
Faustian Phytochem Investigator
 
Posts: 194 Joined: 31-Oct-2011 Last visit: 14-Sep-2023 Location: Oaxaca
|
endlessness wrote:In any case, every DMT I tested had at least DMT and 2MTHBC. This would make one think that 2MTHBC is an artifact of some process along the way. Especially with it not being reported in A. confusa. endlessness wrote:It was also there in big medicine and aq1 phalaris This is almost to be expected as there are multiple lit. refs. for this cmpd in both P. aquatica and arundinacea (Medicine being a possible cross) endlessness wrote:but not in yugo red (Which only showed traces of dmt) Again, not a big surprise. 'G' cultivars of P. arundinacea (assuming that is the Sp. of Yugo Red) don't produce the BCs. 2MTHBC is associated with DMT in both the biosynthetic pathway of 'T' type plants as well as reaction artifacts. IE, when the ring close to from the BC, the dimethly group of DMT becomes the 2M (dimethyl) group of the BC. The fact that 2MTHBC is coming up in trace quantities in all results would seem to indicate that any that is forming in the Yugo Red sample is so small as to be undetectable. Do you have UV-VIZ for J-6 ?? The results posted are a bit surprising from looking at the plate. -D.
|
|
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 06-Feb-2025 Location: Jungle
|
No I dont have UV-VIS for others, I can try to get them done if I still have any of the samples.
Why are the results surprising?
|
|
|

Faustian Phytochem Investigator
 
Posts: 194 Joined: 31-Oct-2011 Last visit: 14-Sep-2023 Location: Oaxaca
|
From looking at the TLC plates, I would have thought #8 would have been the most pure and #7 the least based off of the spot under the DMT spot.... However, looking at them again, it appears that #7 and #9 where more saturated solutions or heavier spotting based on the size of the DMT spot, this is probably what threw me off at first look.
I was really just curious about the other samples, no big deal really.
|
|
|

Faustian Phytochem Investigator
 
Posts: 194 Joined: 31-Oct-2011 Last visit: 14-Sep-2023 Location: Oaxaca
|
endlessness - when you have time, do a comparison of the unknown BC in the #1 unwashed jungle MS against methyl gallate and veratric acid. See if the are a close match or not.
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 06-Feb-2025 Location: Jungle
|
So i've found out why the NMT is not appearing. It basically co-chromatographs with DMT in this column/system we're using. It does appear as separate peaks when NMT is in large quantities (for example in Acacia confusa), but in very small quantity it appears under the curve area of DMT and the AMDIS program doesn't detect it as a separate peak (but if you go manually just before DMT, you can see in the ion scans that there are the two main peaks at 131, 44 and 77 (and the molecular peak at 174). So there you go, indeed those spots under DMT in TLC were NMT Another funny aspect of NMT and DMT co-chromatographing in this system is that in one acacia analysis it appears just before, and in another just after DMT.. Im not enough chem-savvy to explain why this happens but they are similar enough molecules so that it wouldn't be too hard to imagine this happening, considering they are co-chromatographing. By the way dozuki, here are the spectrums of those two substances you asked, I dont think they are the mistery b-carboline.. I dont think either it is the suggested identification from NIST (Cycloheptan[a]indole), because in the mistery substance there is a very strong peak at 200, which is possibly it's molecular weight. endlessness attached the following image(s):  veratricacid.jpg (52kb) downloaded 646 time(s). methylgallate.jpg (51kb) downloaded 647 time(s). mistery bcarbl.jpg (94kb) downloaded 648 time(s).
|
|
|

DMT-Nexus member
Posts: 1453 Joined: 05-Apr-2009 Last visit: 02-Feb-2014 Location: hypospace
|
This is great work!
I'd like to see a method for DMT--->2meTHBC on purpose to better understand the probable artifact.
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 06-Feb-2025 Location: Jungle
|
Indeed AlbertKLloyd. Dozuki mentioned in this Phalaris thread about the Pictet-Spengler reaction. Any suggestions on how we could go about this test? In the Acacia analysis i've done that contained DMT, they also were shown to have 2MTHBC, so Im thinking indeed it might be an artefact of analysis. When we have an LC-MS in the near future, cross-testing these things will be good because LC-MS should not create same artefacts since it doesn't involve the same heat and conditions
|
|
|

DMT-Nexus member
Posts: 1453 Joined: 05-Apr-2009 Last visit: 02-Feb-2014 Location: hypospace
|
I've been looking into Lewis acid pictet spengler reactions wondering if there is something to this. I could be barking up the wrong tree on this. I suspect that with the right lewis acid and right solvent it might be possible to do a microwave mediated lewis acid catalyized pictet spengler reaction on various tryptamines. There is an interesting title out there: Microwave‐Assisted One‐Pot Preparation of Tetrahydro‐β‐carboline Hydrochlorides under Solvent‐Free Conditions Quote:Abstract
An efficient and environmentally friendly synthesis of tetrahydro‐β‐carboline hydrochlorides via Pictet–Spengler reaction was described. Tryptamine hydrochlorides were used as the reactant and no additional acid catalyst was needed. This reaction was completed within 2.5–9 min in good yield. I don't have access to the full paper but would very much like to read this one. I think it might be of use here. Sorry if this is a bad lead.
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 06-Feb-2025 Location: Jungle
|
So i've sent some crude mimosa methanol soak to analyze. Here are the results: 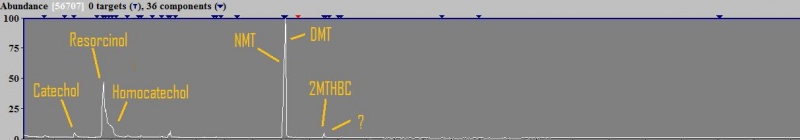 So main surprise are these phenol-based substances, catechol, homocatechol and resorcinol, which I read are quite common substances and might be what are responsible for the skin-healing effect of mimosa. There are other peaks which I cannot identify due to weak signal (and one seems the hordenine, a contamination from previous sample which was a phalaris, since the person running the samples told me they had not added a blank between those samples ). Also, again together with the 2MTHBC there is this mistery substance (edit; pretty sure it is 1,2-dimethyl-tetrahydrobetacarboline, check post further down). Interesting MTHBC itself isnt in mimosa methanol soak, so its probably being created during extraction of Mimosa because it does appear in jungle spice and FASW. Im also attaching the .MS file as always
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 06-Feb-2025 Location: Jungle
|
Thanks AlbertKLloid! Im attaching the paper you mentioned
|
|
|

Faustian Phytochem Investigator
 
Posts: 194 Joined: 31-Oct-2011 Last visit: 14-Sep-2023 Location: Oaxaca
|
@Albert - no, I think that looks like a good lead and I too would love to read that paper. The only solid things I've found relate to tryptamine and serotonin going through ring closure to become beta-carbolines, and these tryptamines are slightly different in structure to DMT or 5-MeO having an amine group where the dimethyl group of latter compounds are. Wikipedia states: "The original Pictet–Spengler reaction was the reaction of β-phenethylamine with the dimethyl acetal of formaldehyde and hydrochloric acid forming a tetrahydroisoquinoline." And further: "It is the electrophilicity of the imine double bond that is the driving force of the cyclization." From: [7] Cox, E. D.; Cook, J. M. (1995). "The Pictet-Spengler condensation: a new direction for an old reaction". Chemical Reviews 95 (6): 1797–1842. Which looks like another good reference to track down. As for procedural this is from "The xanthydrol reaction for pyrroles and indoles in histochemistry: zymogen granules, lens, enterochromaffin and melanins" R.D. Lillie (1956) Its a print out of a PDF copy, not sure of the origin of publication: P.191: "Effects of formaldehyde: 200mg tryptamine was dissolved in dH2O with 200mg calcium acetate, the volume brought to 18cc with water and 2cc 37% formaldehyde was added. Opalescence appeared in a few seconds and a copious white precipitate formed. Which remained quite well suspended." It goes on to give how they seperated that out with a centrifuge and and test this in xanthydrol giving a "somewhat retarded purple black reaction". They don't specifically state what this reaction is checking for other than there naturally was formaldehyde in tissue samples. P.192: "Barter and Pearse have explained the negative Ehrlich's reaction of enterochromaffin on the basis that 5-hydroxytryptamine, condensed with formaldehyde to 6-hydroxytetrahydroharman..." The rest of the info I've found is more casual reference to this ring closure in Shulgin, and maybe one other place. @endlessness - Thanks for attaching that paper, I will have a good look at it. Any chance of finding the one mentioned here? Also, I'm not surprised at the phenols in the methanol soak. Look at catechol and the others and then at yuremamine. These phenol are, or form the basis of the tannins in M. hostilis. I'm surprised there weren't more of them. Very interesting about the NMT, looks like you were correct there =) I'm really not sure on the mystery compound. You are doing a great job identifying thus far. endlessness wrote:...because in the mistery substance there is a very strong peak at 200, which is possibly it's molecular weight. Yea, as I understand it, the highest # peak is the M+ which is the molecular weight, and that the highest peak is the cmpds 'base' peak. As for the compounds I asked about above, they are phenols that have been found in M. hostilis or are close similarly structured phenols that also have some similarity to the phenol attachments on yuremamine.
|
|
|
member for the trees
  
Posts: 4003 Joined: 28-Jun-2011 Last visit: 27-May-2024
|
..NMT (from previous experiments) was less soluble than DMT in cold ethanol..i wonder if during co-crystalization some kind of polymorphic crystal lattice is created, giving slightly different results..
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 06-Feb-2025 Location: Jungle
|
Thanks for the posts! Dozuki, im attaching the paper you mentioned ( Cox, E. D.; Cook, J. M. (1995). "The Pictet-Spengler condensation: a new direction for an old reaction". Chemical Reviews 95 (6): 1797–1842. ) . If you get any information of interest, let us know. What you mentioned about the pictet spendler of phenetylamine forming a quinoline compound makes me wonder if the quinoline compound found in acacia analysis, specially of Acacia acuminata, could be an artifact from the phenetylamines found being cyclicized? Also thanks for the tannin info, didn't know those phenols are what compose the tannins! nen, what do you mean with "giving slightly different results"? In a GC-MS ? Or the looks of the crystallized product? Or... ? Also im quoting you from the jungle spice analysis thread: nen888 wrote:..an experienced chemist told me: " In the case of NMT, oxidation and cyclization would give 1,2,3,4-tetrahydro-beta-carboline."
.. hmmmm, interesting, thats = tryptoline, right? .. And what would dmt cyclicize to?
|
|
|

Faustian Phytochem Investigator
 
Posts: 194 Joined: 31-Oct-2011 Last visit: 14-Sep-2023 Location: Oaxaca
|
Thank you for the paper, I read through the parts that would be useful to this discussion, but I think that one is a dead end as they were modifying the reaction and that modification didn't work with tryptamine. I did pull some more references last night and will post pertinent info once I sort through those refs. As for the quinoline, I had the same thought when looking at the MS that you posted. I also saw @nen's post and it got me wondering what the differences would be when cyclizing tryptamine vs MMT vs DMT. Right now I'm very unclear about it. Like I posted earlier, most of the references start with trypamines having an amine group and I've found no references to the reaction with a methyl or dimethyl group. Shulgin and other references state that tryptamine forms 1,2,3,4-THBC when cyclized using the PS condensation. When I get the info sorted, I will probably post a new topic in this sub-forum as you have to be a member to see this one. Hopefully that will help alleviate concerns that this topic is broaching the synthesis rule. P.S. Tho not directly related, any chances of finding this paper? : Chromatography of Beta-Carbolines
|
|
|
member for the trees
  
Posts: 4003 Joined: 28-Jun-2011 Last visit: 27-May-2024
|
..by 'slightly different results' i was referring to NMT appearing both before and after the DMT in the recent chromatographs.. presumably they are often virtually intermingled due to their similarity..
..i'm curious now as to how plants turn NMT into DMT...
|
|
|

Stiletto Stoner

Posts: 1132 Joined: 18-Nov-2008 Last visit: 15-Mar-2015 Location: Blazin'
|
Got GVG ? Mhm. Got DMT ? Pandora wrote:Nexus enjoys cutting edge and ongoing superior programming skills of the owner of this site (The Traveler), including recent switching to the .me domain name. I'm still, I'm still Jenny from the block Simon Jester wrote:"WTF n00b, buy the $100 vapor pipe or GTFO" Ignorance of the law does not protect you from prosecution
|
|
|

Faustian Phytochem Investigator
 
Posts: 194 Joined: 31-Oct-2011 Last visit: 14-Sep-2023 Location: Oaxaca
|
|
|
|
member for the trees
  
Posts: 4003 Joined: 28-Jun-2011 Last visit: 27-May-2024
|
Quote:1,2,3,4-tetrahydro-beta-carboline (also goes by tryptoline or noreleagnine) is found in these plants:
Commelina communis (Commeliaceae)
Eudistoma glaucus (Ascidiacea)
Lolium perenne (Graminaceae)*
Phalaris arundinacea (Graminaceae)**
Strychnos johnsonii (Loganiaceae)***
Testulea gabonensis (Ochanaceae) trunkbark
Villagorgia rubra from TIHKAL, pp 683-684 ..it has been theorized to be a possibly very potent MAOI to explain phalaris staggers as sheep 'ayahuascaing themselves'.. .
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 06-Feb-2025 Location: Jungle
|
Thanks to nen's heads up in the acacia analysis thread, Im pretty positive the last mistery b-carboline we've been seeing in jungle spice is 1,2-Dimethyl-Tetrahydrobetacarboline (or.. 1,2-dimethyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole ) 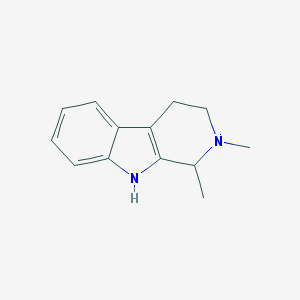 It IS also present in the simple mimosa methanol soak, so it is not an artifact of extraction, though it might be of analysis, im not sure... We need someone with more knowledge with GC-MS (or wait for LC-MS tests in about a month) to answer this question
|
|
|

Faustian Phytochem Investigator
 
Posts: 194 Joined: 31-Oct-2011 Last visit: 14-Sep-2023 Location: Oaxaca
|
I did some digging around on an old hard drive and found images of the last plate I ran on MHRB. The purple lanes are longwave UV. Lane 1 is a standard A/B extraction using either toulene, xylene, or naptha as the non polar. Lanes 2 and 3 are a bit more complicated: The same material was soaked in MeOH for 1 hour at room temperature and done twice. This extraction was then treated with gelatin and freeze/thawed a couple of times to try and remove the tannins.This precipitated a large amount of the red tannins and then also precipitated a blue compound that at the time I thought was a different color tannin. I'm not so sure about this now. That compound I believe is the blue spot 'E' in plate 18 that I posted earlier. Once the gelatin treatment was done, it was extracted with CHCl3 to try and further separate the compounds, which it did to a degree. Lane 2 is the MeOH layer after extraction with CHCl3 and Lane 2 is the CHCl3 layer. The plate is visualized with xanthydrol and ran IPA:NH4OH:H2O What is interesting here is that there appears to be 9-10 compounds including the ones that only show up under UV. Also, spot #1 in Lane #1 is the spot that disappears after 4 days in an A/B extraction. It also shows up as Spot #1 in lane 3. Being at the top of the plate. I've also included the pre-visualized plate in UV for comparison. Dozuki attached the following image(s):  20.jpg (107kb) downloaded 437 time(s). 20UV.jpg (104kb) downloaded 433 time(s).
|