
DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 24-Apr-2024 Location: Jungle
|
As inf said  Also, as another illustrative example, take bufotenine vs psilocin. Psilocin is 4-Hydroxy-N,N,DMT, or 4-HO-DMT, while bufotenine is 5-Hydroxy-N,N-DMT, or 5-HO-DMT. Now look where the hydroxy group of both is located. Essentially they are what is called positional isomers, where the molecule is basically the same, but one functional group (in this case the hydroxy) is located in another part of the molecule.  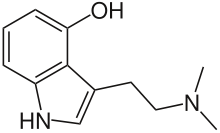
|
|
|
|
|
DMT-Nexus member
Posts: 47 Joined: 25-Dec-2012 Last visit: 12-Jan-2017
|
When using a weak acid during an A/B extraction, is there a possibility of the DMT Salt part of the reaction reversing?
Such that when the defat stage is applied, some freebase DMT is accidentally extracted and wasted?
Or is it that once the weak acid reacts to form the DMT salt that it stays a salt?
|
|
|

analytical chemist
   
Posts: 7463 Joined: 21-May-2008 Last visit: 03-Mar-2024 Location: the lab
|
It is possible, but it would have to be a really weak acid, pH > 5. "Nothing is true, everything is permitted." ~ hassan i sabbah
"Experiments are the only means of attaining knowledge at our disposal. The rest is poetry, imagination." -Max Planck
|
|
|
DMT-Nexus member
Posts: 47 Joined: 25-Dec-2012 Last visit: 12-Jan-2017
|
Im referring to the acid being weak, as in the reaction being a reversible reaction. Rather than the pH being low.
Weak acids that create reversible reactions would in theory produce lower yields when a defat is performed, wouldnt they?
|
|
|

analytical chemist
   
Posts: 7463 Joined: 21-May-2008 Last visit: 03-Mar-2024 Location: the lab
|
it's acid/base equilibria; protonated/deprotonated functional groups.. in the case of alkaloids, the amines. in the acidic range (pH < 7), DMT is >99% protonated. this is constant, not reversible. you shift the equilibrium by adding base. "Nothing is true, everything is permitted." ~ hassan i sabbah
"Experiments are the only means of attaining knowledge at our disposal. The rest is poetry, imagination." -Max Planck
|
|
|
DMT-Nexus member
Posts: 559 Joined: 24-Dec-2011 Last visit: 03-Nov-2020
|
When you add sodium hydroxide to water I know it is an exothermic reaction. But if you make a solution of lye and water and then and that add the resulting basic solution to acidified water will it produce that same exothermic reaction? Or do you only get the heat when you initially combine the water/lye?
|
|
|

analytical chemist
   
Posts: 7463 Joined: 21-May-2008 Last visit: 03-Mar-2024 Location: the lab
|
there will always be heat released when you shift the equilibrium (also entropy of mixing), but in this case, it's not as exothermic as dissociation of hydroxide anions and sodium cations from lye in water. "Nothing is true, everything is permitted." ~ hassan i sabbah
"Experiments are the only means of attaining knowledge at our disposal. The rest is poetry, imagination." -Max Planck
|
|
|
DMT-Nexus member
Posts: 559 Joined: 24-Dec-2011 Last visit: 03-Nov-2020
|
So it'll still get hot, but not as hot. More or less what I figured, thanks.
|
|
|

DMT-Nexus member
Posts: 345 Joined: 05-Sep-2013 Last visit: 06-Nov-2015
|
Is the formation of acetate salts of alkaloids reversible or irreversible? If the former is the case, how would one push the equilibrium towards the maximum amount of salts? Thanks edit - And are acetate salts of alkaloids 100% insoluble in toluene? My avatar was taken from google images and is actually a work of art by NEIL GIBSON, credit where credit is due! Bodies don't have souls - souls have bodies Old enough to know better, young enough to try again
|
|
|

Kalt und Heiß, Schwarz und Rot, Kürper und Geist, Liebe und Chaos
 
Posts: 4661 Joined: 02-Jun-2008 Last visit: 30-Apr-2022
|
Formation of any salt of alkaloid is reversible and by manipulating ph you can swich from freebase to salt to freebase virtually forever... For maximum amouny of salt formation you just need a low enough ph. The pka value of each alkaloid determines how much is going to be salt or freebase at a given ph value. Or to be more precise, since salts of alkaloids anyway do not exist when dissolved in solution, the ph determines how much of a given alkaloid will be freebased or ionised. As for dmt acetate, i do not think someone has determined iys solubility in toluene. "Dry" dmt acetate may be soluble in toluene to a small extent. dmt acetate dissolved in water may have a ph that favors dmt freebasing, so some of it may be soluble in toluene. But dmt acetate dissolved in water in the presence of enough additional acid to keep it acidic, will not favor any dmt freebase species and will not be soluble in toluene. Need to calculate between salts and freebases? Click here! Need to calculate freebase or salt percentage at a given pH? Click here!
|
|
|

DMT-Nexus member
Posts: 345 Joined: 05-Sep-2013 Last visit: 06-Nov-2015
|
Thank you, how you explain it is how I understood things so far, but... When enough freebase alks are dissolved in the toluene, it would make the toluene slightly basic - the amount of vinegar that seems to be necessary to salt the alks out of the toluene is way bigger than the amount of vinegar that would be needed to dissolve the same amount of alkaloid acetates (in the absence of "basic toluene"?), so I conclude that vinegar is not acidic enough for this purpose... Or am I overestimating the basic pH of the toluene when "full" of freebase alks? I can't wrap my head around the reason why it takes so much vinegar to salt out whatever small amount of alks may be in the toluene (comes from mhrb that had already yielded 1.68% with naphtha, so there can't be that many alks in there)... I also understand that non polar solvents have no pH due to being immiscible with water...however I also understand that this immiscibility is never 100% - so could an nps "acquire" a pH after contact with for instance basic soup? My avatar was taken from google images and is actually a work of art by NEIL GIBSON, credit where credit is due! Bodies don't have souls - souls have bodies Old enough to know better, young enough to try again
|
|
|

DMT-Nexus member
Posts: 345 Joined: 05-Sep-2013 Last visit: 06-Nov-2015
|
Okay, this has been baffling me for long enough now... It has been stated in various threads by Nexians whose knowledge of chemistry is acknowledged by us all that contamination with plastic during crystal formation is very unlikely because when forming a crystal the molecules are quite xenophobic (they don't like any "strangers" around)... So any contamination with plastic should be very minimal/negligible... On the other hand it has been stated by equally respected members that crystals formed in basic water will be contaminated with traces of base... How can both these claims be true at the same time? Shouldn't any contamination be on the crystals surface rather than being embedded in it and shouldn't one be able to simply rinse it off? Why is recrystallisation deemed necessary? The only thing I could think of is that molecule size or shape is a factor, allowing some contaminants to enter the crystal lattice while others would be excluded from it - but that's just a wild guess  Could someone please clarify this? I'm suffering from the sort of confusion that isn't enjoyable  lol PLUR ps It would be nice if benzyme and Dr Sister could chime in - they're the ones who caused this confusion lol My avatar was taken from google images and is actually a work of art by NEIL GIBSON, credit where credit is due! Bodies don't have souls - souls have bodies Old enough to know better, young enough to try again
|
|
|
novelty junkie extraordinaire
Posts: 28 Joined: 12-Dec-2013 Last visit: 10-Jan-2014
|
I just want to peek my head in and say that Khan Academy is a GREAT resource if you're interested in learning a bit about chemistry. They cover everything from the fundamentals of chemistry to some pretty advanced Organic chemistry.
Also, could someone please explain to me the rules regarding discussion of synthesis on this site? I know that now discussion that includes "dangerous chemicals" is allowed, but what about eco-friendly, safe, total synthesis?
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 24-Apr-2024 Location: Jungle
|
dooby wrote:Thank you, how you explain it is how I understood things so far, but...
When enough freebase alks are dissolved in the toluene, it would make the toluene slightly basic - the amount of vinegar that seems to be necessary to salt the alks out of the toluene is way bigger than the amount of vinegar that would be needed to dissolve the same amount of alkaloid acetates (in the absence of "basic toluene"?), so I conclude that vinegar is not acidic enough for this purpose... Or am I overestimating the basic pH of the toluene when "full" of freebase alks?
I can't wrap my head around the reason why it takes so much vinegar to salt out whatever small amount of alks may be in the toluene (comes from mhrb that had already yielded 1.68% with naphtha, so there can't be that many alks in there)...
I also understand that non polar solvents have no pH due to being immiscible with water...however I also understand that this immiscibility is never 100% - so could an nps "acquire" a pH after contact with for instance basic soup? You don`t need much vinegar or other acids to salt out a non polar solvent full of alkaloids. Do 3x saltings with small amounts of acidic solution.. If you measure the pH of the solution, it starts acidic but can get more basic after mixing with the non-polar, but the second or third saltings already stays more acidic. dooby wrote:Okay, this has been baffling me for long enough now...
It has been stated in various threads by Nexians whose knowledge of chemistry is acknowledged by us all that contamination with plastic during crystal formation is very unlikely because when forming a crystal the molecules are quite xenophobic (they don't like any "strangers" around)... So any contamination with plastic should be very minimal/negligible...
That is not correct. The presence or lack of plastic depends whether you used plastic and a solvent that dissolved plastic in the first place. If you did, then you will have plastic or plasticizers in your product one way or another. Recrystallization or for example FASA will help get rid of that because the plasticizers will stay in the solvent and you harvest the crystals. Washing is a more superficial way of cleaning substances, and the bigger the crystals, the worse you will clean inside of it. It has been reported many times about crystals having foreign substances within it's crystal lattices, such as for example solvents and others. So work clean, and do recrystallizations and reprecipitation/washes, if you want the best product. JohnGriggsII wrote:
Also, could someone please explain to me the rules regarding discussion of synthesis on this site? I know that now discussion that includes "dangerous chemicals" is allowed, but what about eco-friendly, safe, total synthesis?
As written in the attitude section, synthesis of material that does not use watched or dangerous chemicals or proceedures, and that is for personal consumption, are ok to discuss here.
|
|
|
DMT-Nexus member
Posts: 20 Joined: 28-Jan-2014 Last visit: 02-Apr-2014 Location: Jersey
|
If acid to base is more useful than straight to base, can someone explain why a ph of 4 to 13 is more useful than say, ph of 1-2 to 13? Or is it true that a PH of 1 is better than 4 because there is a greater difference?
Can someone explain exactly what polar/non-polar solvents are?
Does anyone have a list of non-polar solvents? What makes vm&p naptha better or worse than n-heptane?
Is slow evaporation of non-polar solvent better than normal? In other words, would it be more desirable to evaporate in fridge>room temperature? what about room temperature with ambient light> room temperature in complete darkness?
Are there any known steps where light/darkness effect the procedure?
|
|
|

DMT-Nexus member
Posts: 415 Joined: 10-Jul-2010 Last visit: 18-Apr-2020 Location: Earth
|
ScriabinAnime wrote:If acid to base is more useful than straight to base, can someone explain why a ph of 4 to 13 is more useful than say, ph of 1-2 to 13? Or is it true that a PH of 1 is better than 4 because there is a greater difference?
Can someone explain exactly what polar/non-polar solvents are?
Does anyone have a list of non-polar solvents? What makes vm&p naptha better or worse than n-heptane?
Is slow evaporation of non-polar solvent better than normal? In other words, would it be more desirable to evaporate in fridge>room temperature? what about room temperature with ambient light> room temperature in complete darkness?
Are there any known steps where light/darkness effect the procedure? I can answer some of these inquiries. pH is a measurement of the concentration of Hydrogen ions in a solution. One lowers the pH to around 4 in order to make soluble your DMT molecule in water, this way your molecule remains behind in the water as opposed to leaving with your defat, nonpolar solvent when you are removing the plant fats in solution. The molecule is made soluble by protonation or addition of a proton, and pH 4 is the optimal pH for keeping this molecule protonated. pH 1 would be more protons than needed, so to speak. A polar solvent would be water. A nonpolar solvent or chemical would be vegetable oil, other fats or hydrocarbons. Put simply, it is a difference in the way electrons are shared across a molecule. Polar molecules have uneven sharing of electrons that results in a difference in partial charge across the molecule. Water has a concentration of electrons about the oxygen and a lack of electrons about it's two hydrogens. Nonpolar molecules have relatively even sharing of electrons across molecules. Nonpolar and polar solvents do not like to mix: water and oil being the classic example. Certain molecules will dissolve much easier in certain solvents based on their similar polarities/charge distribution. With dmt, we essentially use the acid and base steps to make dmt polar or nonpolar by adding and removing these protons and changing the charge across the molecule. In this way we can separate it from the other substances in the bark/plant matter. Living to Give
|
|
|

DMT-Nexus member
Posts: 699 Joined: 06-Jul-2012 Last visit: 20-Dec-2018
|
cave paintings wrote:I can answer some of these inquiries. pH is a measurement of the concentration of Hydrogen ions in a solution. One lowers the pH to around 4 in order to make soluble your DMT molecule in water, this way your molecule remains behind in the water as opposed to leaving with your defat, nonpolar solvent when you are removing the plant fats in solution. The molecule is made soluble by protonation or addition of a proton, and pH 4 is the optimal pH for keeping this molecule protonated. pH 1 would be more protons than needed, so to speak. Is there a pH at which the fats/oils dont migrate to the NPS? I dont know why but it seems that my oils never absorb into the NPS during a defat step. My NPS stays clear, and the acid solution stays yellow. "I am cursed by the blossoming knowledge of my feminine ideal and she looks suspiciously like you."
"Everybody is a genius. But if you judge a fish by its ability to climb a tree, it will live its whole life believing that it is stupid." -AE
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 24-Apr-2024 Location: Jungle
|
No, you shouldn`t smoke alkaloids in their salt form.
In some cases it may be active but generally it is either significantly less eficient than smoking the freebase, or even possibly irritating/toxic. Smoking fumarates for example can lead to the formation of maleic anhydride which can be very irritating to mouth/lungs..
In short: Freebase to smoke, salt for storage or other methods of administration.
|
|
|

DMT-Nexus member
Posts: 1893 Joined: 18-Jan-2008 Last visit: 26-Sep-2023
|
Why does a solvent sometimes attract alkaloids to tje surface yet not dissolve them even if its not saturated?
Is it something to do with the ammount of the base liquid and NP solvent?
|
|
|

DMT-Nexus member
Posts: 699 Joined: 06-Jul-2012 Last visit: 20-Dec-2018
|
DreaMTripper wrote:Why does a solvent sometimes attract alkaloids to tje surface yet not dissolve them even if its not saturated?
Is it something to do with the ammount of the base liquid and NP solvent? Are you meaning to say that your NPS is bringing your dmt to float on the basic solution but not absorb into the NPS? If it is floating there then for some reason or another, the NPS that is in it has become saturated. Most of the times you see floating crystals is from having your NPS sit in the basic solution for too long and it grows cool and the crystals precip out onto the surface of the basic solution. Id say to just try heating up your jar to see if it aborbs into the solvent if warmed. If it doesnt at all, then it isnt DMT. If you want, put in new NPS and heat that. That should guarantee you the ability to know whether its dmt or not. If it is, then that means your original solvent actually was saturated completely. "I am cursed by the blossoming knowledge of my feminine ideal and she looks suspiciously like you."
"Everybody is a genius. But if you judge a fish by its ability to climb a tree, it will live its whole life believing that it is stupid." -AE
|