
DMT-Nexus member
Posts: 267 Joined: 09-Mar-2012 Last visit: 31-Dec-2022
|
Could you please explain the mechanics of temperature in chemistry? I think understanding how and why to use temperature is as fascinating as knowing what pH means.
My current knowledge is that things expand when they're heated and condense when cooled. Any more general information would be appreciated; some specific questions that come to mind are:
How does heating solvent allow more dmt into solution, and why does freezing precipitate?
Is there some scale like pH where I can push the equilibrium of the precipitate more and more by deeper freezing?
How is freebase made from heating and dissociating a salt?
How do you represent the addition of heat in a formula?
Thanks for spreading the magic dudes
|
|
|
|
|
DMT-Nexus member
Posts: 5 Joined: 18-Dec-2011 Last visit: 23-Apr-2012 Location: toronto
|
i want to know something. why does the naptha not pull dmt out of the solution during the defat stage if it so happens to in the extraction stage?
|
|
|

DMT-Nexus member
Posts: 64 Joined: 25-Apr-2012 Last visit: 29-Apr-2015 Location: The Void
|
marcs_the_man wrote:i want to know something. why does the naptha not pull dmt out of the solution during the defat stage if it so happens to in the extraction stage? This is because the DMT in the plant material is bound in form of a salt. Salts are very polar (the amine of the DMT has a positive charge and the anione of the salt has a negative charge) and therefore not soluble in aliphatic hydrocarbons (Naptha). However fatty acids and fat are soluble. Icon wrote:Could you please explain the mechanics of temperature in chemistry? I think understanding how and why to use temperature is as fascinating as knowing what pH means.
My current knowledge is that things expand when they're heated and condense when cooled. Any more general information would be appreciated; some specific questions that come to mind are:
How does heating solvent allow more dmt into solution, and why does freezing precipitate?
Is there some scale like pH where I can push the equilibrium of the precipitate more and more by deeper freezing?
How is freebase made from heating and dissociating a salt?
How do you represent the addition of heat in a formula?
Thanks for spreading the magic dudes Without getting into to much detail (a whole section of chemistry is looking at these phenomena) I wanted to say: Temperature moves molecules. This is very important to know. Solid materials get bigger because the molecules in the metal/ionic-crystal move and wiggle around and the distance between them gets bigger. Solid materials melt because the resistance of the molecules gets less and less the more you heat them (they can move more easily) and finally liquids boil because they can escape the molecule union of the liquid (and fly around free like birds in the vast space of the atmosphere). For solutions this becomes more difficult and we need to take into account the enthalpy. Enthalpy basically says if it is energetically good or bad considering heat to do something. For instance: You put a salt into water: the water cools. In this case it is endothermic and the Enthalpy of solubility is greater than 0 kJ/mol. If it gets warm it's the opposite. So now on high temperatures endothermic soluble substances are more soluble than exothermic soluble substances (there is more heat they can use for getting in solution). If you freeze them the energy (in form of heat) that they need to stay in solution isn't there so they precipitate. This has to do with the Crystal-energy, Hydratation energy, and can sometimes be calculated. For many subtances the solubility is higher at higher temperatures but this differs from substance to substance and from solvent to solvent. For example you can have a substance that is basically not soluble in boiling water but is very soluble in water that has 3-4°C.
|
|
|
DMT-Nexus member
Posts: 13 Joined: 24-Apr-2012 Last visit: 22-May-2012
|
This thread has been very informative and useful. I have a question, or request rather about applying information I have learned here. I assume since it is basic chemistry, there are other instances which are basically the same as processes used for DMT. NOTE: This will be my very first foray into chemistry since 8th grade science class - and basically I don't remember squat from that. Would it be possible for someone to present a 'PRACTICE' experiment. Something maybe involving household things, salt, milk water, OJ whatever. Where the process will be fundamentally the same and I can see it in action before trying with the real things. As I understand it there are a variety of methods of extracting or using DMT. I'm interested in the smoking form - So a process similar to that with tangible results would be great. As well as the tea. I don't want to sound dense, I know about making tea and am able to follow instructions but I really want to feel familiar with this process and I learn best hands on. I really hope I made sense with my request.  
|
|
|

DMT-Nexus member
Posts: 64 Joined: 25-Apr-2012 Last visit: 29-Apr-2015 Location: The Void
|
Yes, for example D-Limonene (http://en.wikipedia.org/wiki/Limonene) can be isolated from dried orange peels (dry in an oven at 150 °C for 4 or 5 hours) by boiling in the threefold amount of CH2Cl2 (Dichlormethane) for 5 hours. Dry your solvent with sodium sulfate and let the CH2Cl2 evaporate and you got it!
Most of these extractions require vacuumdestillation, fancy solvents (CH2Cl2 and Et2O arent as easily available as say: Naptha) and Reflux apparatus. Therefore the DMT extraction is very easy and more doable at home than most other isolations.
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 30-Apr-2024 Location: Jungle
|
velocity wrote:This thread has been very informative and useful. I have a question, or request rather about applying information I have learned here. I assume since it is basic chemistry, there are other instances which are basically the same as processes used for DMT. NOTE: This will be my very first foray into chemistry since 8th grade science class - and basically I don't remember squat from that. Would it be possible for someone to present a 'PRACTICE' experiment. Something maybe involving household things, salt, milk water, OJ whatever. Where the process will be fundamentally the same and I can see it in action before trying with the real things. As I understand it there are a variety of methods of extracting or using DMT. I'm interested in the smoking form - So a process similar to that with tangible results would be great. As well as the tea. I don't want to sound dense, I know about making tea and am able to follow instructions but I really want to feel familiar with this process and I learn best hands on. I really hope I made sense with my request.   You can try mixing some cooking oil with water and separating the oil with a pipette or similar.. that should at least give you an idea of doing the pulls Extractins are very simple, if you do an a/b, its just like making a tea (except you boil 3x instead of one), then you reduce the liquid to manageable amount .. then you add base till its black and do liek I mentioned above, separating the top layer from bottom.. Then evap or freeze, and voila.. If you do other teks like STB, its more messy somehow but even easier, no tea making, add base, add solvent, separate, evap or freeze. And if you use a solvent like limonene, you have to salt out, which isnt any harder than what you just done.
|
|
|
DMT-Nexus member
Posts: 13 Joined: 24-Apr-2012 Last visit: 22-May-2012
|
Thanks for that idea about the cooking oil and water. I am absolutely going to do that as I was initially hesitant that I'd be able to get a perfect separation.
I think it's funny with something like this - when you first start out even the most basic instructions look like latin. Then with experience it all starts to make sense. So unfortunately I have no clue what the last half of your post means, which tells me: I have a lot to learn!
Thank you for the tips, I will definitely refer back.
|
|
|

lettuce
Posts: 1077 Joined: 26-Mar-2012 Last visit: 15-Jan-2016 Location: Far, Far Away
|
once you do it a couple times it's no harder than making mac-n-cheese (but waaaay better for you IMHO) Pup TentacleYou are precisely as big as what you love and precisely as small as what you allow to annoy you.Robert Anton WilsonMushroom Greenhouse How-ToI'm no pro but I know a a few things - always willing to help with Psilocybe cubensis cultivation questions.
|
|
|

Psilosopher
Posts: 205 Joined: 30-Jul-2012 Last visit: 28-Nov-2022 Location: International waters
|
This is a really interesting thread, thanks for the informative posts  I need several more read-throughs to make this stick in my mind (I have little to no prior chemistry knowledge at all), but I get the general idea. I'm definitely going to read up on more of these topics before attempting my first extraction. Understanding the how and why of every step will certainly give me a way better chance of succeeding. Thanks for the head start  "The only way to deal with an unfree world is to become so absolutely free that your very existence is an act of rebellion." - Albert Camus
|
|
|
DMT-Nexus member
Posts: 44 Joined: 02-Nov-2011 Last visit: 30-Dec-2016
|
i don't think these have been mentioned earlier in this thread after reading.. (please correct if mistaken) 1. PHwhile lowering the PH of a MHRB-water mixture, one reaches a certain PH value with a certain ratio between amounts of used base and water Q: does time influence this PH value ? i understand the PH drop during a naphta pull, but what happens to the H+ when the base is binding with other compounds and attacking cellstructures and so forth ? on first impulse i would logically assume a gradual PH drop would take place in a “NaOH-H2O-plant materials" mixture but then looking back at my knowledge of electricity, i would presume eventual occurance of 'loss' of electrical charge, should be impossible if it's not losing any compounds (watervapour, dmt freebase) Q: so what happens with the H+'s during all these different chemical processes ?  if the H+ amount remains the same, how do cell structures get demolished then (basically/roughly) ?
2. Solubilitya solution is a sort of "electrically driven fabric (or 'mutual diffused state'  " which keeps 2 compounds spatially and proportionally spread equally between eachother a polar solvent is called polar because it contains a negative and positive charged side, which each attract and surround their opposite form of another compound, an opposite form which can be found or 'made' (?) Q:is this the H+ & OH- <-> HOH thing ? or does HOH have this polar behaviour because the electrical configuration of (2x)H on 1 side standing opposite to that of O the other ? i see that most non-polars mainly have Hydrogen on the outside Q: so is this molecular configuration what basically explains the principle of non-polarity ? or what does ? Q: how does a non-polar dissolving work ? what takes care of the equality in proportional and spatial spreading of both substances ? now acetone has different compounds that are soluble in it than the compounds water has Q:if a fraction of water would be dissolved in acetone, how would this influence the solubility properties of the slightly impure acetone ? as in, what is it that a few water molecules, spread over millions of acetone molecules does, substantially ?
|
|
|

Kalt und Heiß, Schwarz und Rot, Kürper und Geist, Liebe und Chaos
 
Posts: 4661 Joined: 02-Jun-2008 Last visit: 30-Apr-2022
|
Kryll wrote:i don't think these have been mentioned earlier in this thread after reading.. (please correct if mistaken) 1. PHwhile lowering the PH of a MHRB-water mixture, one reaches a certain PH value with a certain ratio between amounts of used base and water Q: does time influence this PH value ? i understand the PH drop during a naphta pull, but what happens to the H+ when the base is binding with other compounds and attacking cellstructures and so forth ? on first impulse i would logically assume a gradual PH drop would take place in a “NaOH-H2O-plant materials" mixture but then looking back at my knowledge of electricity, i would presume eventual occurance of 'loss' of electrical charge, should be impossible if it's not losing any compounds (watervapour, dmt freebase) Q: so what happens with the H+'s during all these different chemical processes ?  if the H+ amount remains the same, how do cell structures get demolished then (basically/roughly) ? Generally, time influences pH value but that is negligible. What time does is that it allows for things to dissolve/homogenise. NaOH does not dissolve instantly, it takes some time and while it dissolves pH raises. Equally, as the bark is slowly eaten up (i.e. in an STB exraction) NaOH is "consumed" and pH will drop. Or if any carbon dioxide dissolves in teh mixture it will by time neutralise a small part of NaOH. Now, in the two latter scenarios the dropping of pH is really, for all practical reasons, negligible. I am talking about pH drops by 0.01-0.001 degrees (that is a guesstimate) Also, pH does not drop "during a naphtha pull", unless you mean during "mhrb being in a basic solution", in which case this is explained in the above paragraph. Kryll wrote:2. Solubilitya solution is a sort of "electrically driven fabric (or 'mutual diffused state'  " which keeps 2 compounds spatially and proportionally spread equally between eachother a polar solvent is called polar because it contains a negative and positive charged side, which each attract and surround their opposite form of another compound, an opposite form which can be found or 'made' (?) Q:is this the H+ & OH- <-> HOH thing ? or does HOH have this polar behaviour because the electrical configuration of (2x)H on 1 side standing opposite to that of O the other ? i see that most non-polars mainly have Hydrogen on the outside Q: so is this molecular configuration what basically explains the principle of non-polarity ? or what does ? Q: how does a non-polar dissolving work ? what takes care of the equality in proportional and spatial spreading of both substances ? as in, what is it that a few water molecules, spread over millions of acetone molecules does, substantially ? The dissociation of water (H + OH- <-> HOH) does not account for the polarity of the water. Dissociation of the water is what gives the water the property of pH. As you correctly say, Quote:HOH have this polar behaviour because the electrical configuration of (2x)H on 1 side standing opposite to that of O the other ? Non-polar solvents do not exactly have "Hydrogen on the outside" they have hydrocarbons. Naphthas for instance are rich in hydrophobic -(CH2)- groups. Quote:
now acetone has different compounds that are soluble in it than the compounds water has
Q:if a fraction of water would be dissolved in acetone, how would this influence the solubility properties of the slightly impure acetone ?
Yes. Need to calculate between salts and freebases? Click here! Need to calculate freebase or salt percentage at a given pH? Click here!
|
|
|
DMT-Nexus member
Posts: 44 Joined: 02-Nov-2011 Last visit: 30-Dec-2016
|
Infundibulum wrote:
Also, pH does not drop "during a naphtha pull", unless you mean during "mhrb being in a basic solution", in which case this is explained in the above paragraph.
different extraction teks i stumbled upon until now state a certain necessity in adding more base to the mixture, after each solvent pull, (because of freebase alkaloid being subtracted from the mixture afaik) that's why this got included in the global view  weird though it isn't then Infundibulum wrote:
Non-polar solvents do not exactly have "Hydrogen on the outside" they have hydrocarbons. Naphthas for instance are rich in hydrophobic -(CH2)- groups.
that looks like i will be needing an extensive amount of chemistry/physics principles to get that into place hehe is there a simple way to explain HOW -or because of what- non-polar dissolution takes place ?  Infundibulum wrote:Quote:
now acetone has different compounds that are soluble in it than the compounds water has
Q:if a fraction of water would be dissolved in acetone, how would this influence the solubility properties of the slightly impure acetone ?
Yes. i sort of expected that to be the outcome, but was more interested in the HOW and WHAT  thanks for all the clarifications and taking effort / spending time for responding ! much appreciated
|
|
|

DMT-Nexus member
Posts: 64 Joined: 25-Apr-2012 Last visit: 29-Apr-2015 Location: The Void
|
Kryll wrote:is there a simple way to explain HOW -or because of what- non-polar dissolution takes place ?  First you need to understand that polar solvents dissolve polar compounds and non-polar solvents dissolve non-polar compounds. Now what exactly is polarity? Well Water is H-O-H (H2O): an oxygen atom which forms 2 single bonds with hydrogen atoms. The oxygen is more electronegative, that means it pulls the electrons from the bond more to his side thus getting a little negative charge (delta minus because electrons have a negative charge). The hydrogens on the other hand have less electrons because they are further away from them thus giving them a small positive charge (delta plus because the proton of the hydrogen is less shielded by the electron). Therefore water is a polar solvent, it has delta minus and delta plus poles in every molecule. The difference of electronegativity of oxygen and hydrogen is about 1.3 but the difference between carbon and hydrogen is a mere 0.3; Because of this none of the atoms has a small charge, the electrons are distributed equally (more or less). This causes the two liquids (for example Hexane and Water) to form two phases instead of mixing (like alcohol and water). Alcohol (Ethaonol) is also a polar solvent, it has an oxygen atom in its structure. Now there are exceptions to this rule (CH2Cl2, CHCl3, Et2O) which I will not explain thoroughly as this would go too far but basically that's all you need to know.
|
|
|

DMT-Nexus member
Posts: 31 Joined: 14-May-2013 Last visit: 18-May-2013 Location: Archuleta Mesa
|
nigl wrote:Kryll wrote:is there a simple way to explain HOW -or because of what- non-polar dissolution takes place ?  First you need to understand that polar solvents dissolve polar compounds and non-polar solvents dissolve non-polar compounds. Now what exactly is polarity? Well Water is H-O-H (H2O): an oxygen atom which forms 2 single bonds with hydrogen atoms. The oxygen is more electronegative, that means it pulls the electrons from the bond more to his side thus getting a little negative charge (delta minus because electrons have a negative charge). The hydrogens on the other hand have less electrons because they are further away from them thus giving them a small positive charge (delta plus because the proton of the hydrogen is less shielded by the electron). Therefore water is a polar solvent, it has delta minus and delta plus poles in every molecule. The difference of electronegativity of oxygen and hydrogen is about 1.3 but the difference between carbon and hydrogen is a mere 0.3; Because of this none of the atoms has a small charge, the electrons are distributed equally (more or less). This causes the two liquids (for example Hexane and Water) to form two phases instead of mixing (like alcohol and water). Alcohol (Ethaonol) is also a polar solvent, it has an oxygen atom in its structure. Now there are exceptions to this rule (CH2Cl2, CHCl3, Et2O) which I will not explain thoroughly as this would go too far but basically that's all you need to know. Thank you Nigl, I was curious about the polarity thing. “You may control a mad elephant; You may shut the mouth of the bear and the tiger; Ride the lion and play with the cobra; By alchemy you may earn your livelihood; You may wander through the universe incognito; Make vassals of the gods; be ever youthful; You may walk in water and live in fire; But control of the mind is better and more difficult.”
― Paramahansa Yogananda, Autobiography of a Yogi
|
|
|

DMT-Nexus member
Posts: 2151 Joined: 23-Nov-2012 Last visit: 07-Mar-2017
|
What is 'salting?' I've seen reference made to it a couple of times in regards to Phalaris teks, as a away to get around the need for multiple de-fats, but I have no idea what it is. I'm a total neophyte when it comes to chemistry, so trying to figure out how all these Teks work is proving to be quite the challenge. Thanks in advance, y'all are awesome  ~ND "There are many paths up the same mountain."
|
|
|

Kalt und Heiß, Schwarz und Rot, Kürper und Geist, Liebe und Chaos
 
Posts: 4661 Joined: 02-Jun-2008 Last visit: 30-Apr-2022
|
Nathanial.Dread wrote:What is 'salting?' I've seen reference made to it a couple of times in regards to Phalaris teks, as a away to get around the need for multiple de-fats, but I have no idea what it is. I'm a total neophyte when it comes to chemistry, so trying to figure out how all these Teks work is proving to be quite the challenge. Thanks in advance, y'all are awesome  ~ND Salting, or salting out is when you convert freebased alkaloids that are dissolved in the non-polar solvent to salts. Pretty much you take your non-polar pull that (especially in the case of phalaris or other leafy material) will be laden with alkaloids and fats and mix it with acidic water; the alkaloids will migrate to the water (they will be salted) and the fats will stay in the non-polar solvent. This way you got rid of the fats and you made the alkaloids cleaner. Need to calculate between salts and freebases? Click here! Need to calculate freebase or salt percentage at a given pH? Click here!
|
|
|

DMT-Nexus member
Posts: 2151 Joined: 23-Nov-2012 Last visit: 07-Mar-2017
|
I have some questions about nomenclature. 1) What does the N,N mean in N,N-DMT? 2) I know that things like 5-Meo-DMT are DMT molecules with a methoxy molecule attached, but what does 5 mean? Thanks Blessings ~ND "There are many paths up the same mountain."
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 30-Apr-2024 Location: Jungle
|
Di-methyl tryptamine, so it by this name you can deduct it's like a tryptamine molecule but with two methyl groups 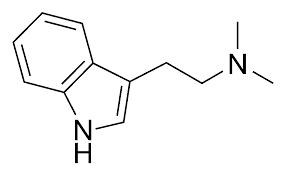 N,N means the two methyl groups are on the nitrogen on that right side. A methyl group is CH3, a carbon atom and 3 hydrogens, but with this kind of drawing, the hydrogen is not shown, it is simply implicit. The lines represent carbon atoms. As for 5-MeO-DMT, the 5 means its on the 5 position in the benzene ring. Here you have a tryptamine molecule with how the positions are numbered:  and below is a 5-MeO-DMT molecule 
|
|
|

DMT-Nexus member
Posts: 2151 Joined: 23-Nov-2012 Last visit: 07-Mar-2017
|
Thanks Endlessness. Just to make sure I got it right: if one of those methyl groups was in the 1 position, you would end up with NH,N-DMT? Blessings ~ND "There are many paths up the same mountain."
|
|
|

Kalt und Heiß, Schwarz und Rot, Kürper und Geist, Liebe und Chaos
 
Posts: 4661 Joined: 02-Jun-2008 Last visit: 30-Apr-2022
|
Nathanial.Dread wrote:Thanks Endlessness.
Just to make sure I got it right: if one of those methyl groups was in the 1 position, you would end up with NH,N-DMT?
Blessings
~ND That one is a bit trickier - you wouldn't call it NH,N-DMT but you could go with rather 1-methyl NMT, or 1-methyl methyltryptamine. Need to calculate between salts and freebases? Click here! Need to calculate freebase or salt percentage at a given pH? Click here!
|