
DMT-Nexus member
Posts: 5826 Joined: 09-Jun-2008 Last visit: 08-Sep-2010 Location: USA
|
SWIM has learned from other SWIMs that dehydrobufotenine is apparently a breakdown product of bufotenine that is sometimes an artifact of extraction especially when heat and excessively high pH values are used during extraction (pH 12 and above). When making Yopo, natives add lime (pH 12.4) and often toast the Yopo. This seems like the prefect recipe for creating dehydrobufotenine from bufotenine. The following clip is from the book TiHKAL #19. 5-HO-DMT: Quote:Dehydrobufotenine: There is a covalent bond formed between the dimethylated nitrogen atom and the indolic 4-position, by the theoretical removal of a molecule of hydrogen. It is no longer a simple tryptamine but as it is a commonly found component of the chemistry of several toads, and a few giant reeds as well, it is included here. It is, by definition, a quaternary amine salt. The original structure assigned it was that of a vinylamine (with the loss of a hydrogen molecule from the alpha/beta chain positions. This was shown to be incorrect. The theory proposed in TiHKAL is that a loss of a molecule of hydrogen causes bufotenine to turn into dehydrobufotenine. In a high pH of about 12, bufotenine should lose a molecule of hydrogen from its hydroxyl group. When this happens to morphine, it forms a salt. But tests done by SWIM's friend show that bufotenine doesn't form a salt at pH 12, but seems to come apart or form another compound. His friend doesn't know what is happening to bufotenine at pH 12, but it is definitely no longer the same molecule. His friend says that before being exposed to pH 12, freebase bufotenine is soluble in acetone, but after being exposed to pH 12 for 24 hours, the result is a compound that is no longer soluble in acetone, but very soluble in alcohol or water. It is also insoluble in non-polar solvents and can't be turned back into bufotenine by the addition of any acids. His friend is unable to verify that it is transformed into dehydrobufotenine, but believes this to be the case. He has not yet tested it for activity. SWIM has done similar tests in the past with inconsistent results. But has on occasion seen what his friend sees. Here's an image of dehydrobufotenine 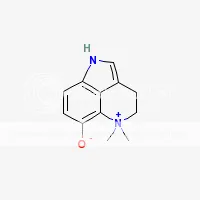 Here's an image of bufotenine 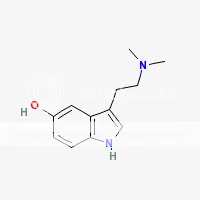 Here is all the information SWIM was able to gather about this alkaloid: Dehydrobufotenine salt (unknown salt in toads) solubility: soluble in methanol, ethanol, water; insoluble in ether, chloroform. Dehydrobufotenine freebase solubility: soluble in water, insoluble in ether. Dehydrobufotenine picrate forms yellow crystals from absolute ethanol. Dehydrobufotenine picrate m.p.: 187 C Dehydrobufotenine picrate solubility: soluble in ethanol Dehydrobufotenine hydrochloride m.p.: 240-244 C Dehydrobufotenine activity: lacks the pressor activity of bufotenine. Judging by the above information, SWIM believes his friend is actually making dehydrobufotenine by simply leaving bufotenine in pH 12 solution for 24 hours. The reason being, freebase bufotenine is soluble in ether, but after sitting in pH 12 solution, the resulting compound is insoluble in ether just like dehydrobufotenine is. The only solvents his friend found that the freebase compound was soluble in after being in pH 12 solution were alcohol and water (just like dehydrobufotenine). It was no longer soluble in many solvents freebase bufotenine is soluble in (acetone, ether, DCM, chloroform) so it can no longer be bufotenine. His friend has not tested the melting points yet. SWIM wants to test the activity of this compound, but doesn’t know how to positively identify it as dehydrobufotenine. What SWIM would like to know specifically about dehydrobufotenine are the following: 1) Is it hallucinogenic? If so, at what doses (oral, vaporized, sublingual, snuffed, etc.)? 2) What solvents is it soluble in as freebase? 3) What color is it as freebase? (Freebase bufotenine is yellow) 4) Is it a solid or an oily substance as freebase? 5) What are it’s freebase melting and boiling points? You may remember me as 69Ron. I was suspended years ago for selling bunk products under false pretenses. I try to sneak back from time to time under different names, but unfortunately, the moderators of the DMT-Nexus are infinitely smarter than I am.
If you see me at the waterpark, please say hello. I'll be the delusional 50 something in the American flag Speedo, oiling up his monster guns while responding to imaginary requests for selfies from invisible teenage girls.
|
|
|
|
|

DMT-Nexus member
    
Posts: 1367 Joined: 19-Feb-2008 Last visit: 12-Jun-2016 Location: Pacific Northwest
|
Over on a more obscure herbal messageboard, in response to the "calcium bufotenate" issue, I found the following: Quote:OK
5 HO-DMT Bufotenine to 5 O-DMT' or Ca+ 5 O-DMT as a compound'
Calcium hydroxide does not bond with Bufotenine'
“Swim took one gram of bufotenine with one gram of calcium hydroxide in a little water to make a paste'
This paste was left at room temp for 14 hours' It had almost dried'
Swim then took the paste and dissolved it in acetone'
The calcium hydroxide falls out of the acetone and pressipitates at the bottom'
The mole we are seeking dissolves in the acetone'
Swim evaped the acetone and got back 940mg of the new mole' don't know of where the 60mg went' maybe left in the sludge at the bottom of the flask'
Swim vapourised 20mg
It is not bufotenine or Calcium Bufotenate' there is no calcium in the final product cos it stays in the bottom of the flask insoluble in acetone'
Prickly sensation' like Bufo' much stronger though'~
After 3 minutes the room is collidoscoping'~
The peak in about ten minutes' the whole experience about two hours'
Extremely visual and euphoric'
The 5-HO-DMT (bufotenine) react with the calcium hydroxide, to form a compound Ca + 5 O-DMT (calcium bufotenate), but splits in the acetone solution leaving 5-O-DMT in solution'
So basically calcium bufotenate only exists in solution' or as of when Bufotenine is in physical contact with calcium hydroxide' as a compound'
Calcium Bufotenine/nate is only existent within the reaction'
The end product is 5 O-DMT'~
As in the snuff' the calcium does not enter the blood stream' but the 5 O-DMT does' so the compound calcium Bufotenine/Bufotinate does not exist as a tryptamine molecule' only as a compound of the tryptamine Bufotenine and calcium hydroxide' Ca+ 5 O-DMT'
The calcium hydroxide knocks out the H' from the 5 HO-DMT' leaving 5 O- DMT'~
Take the seeds' roast them' split them' grind the pip into a fine dust' mix it's own weight with the same amount of Calcium Hydroxide' and a little water into a paste' leave to stand until the acrid fishy smell has gone'
Take the paste and dissolve it in acetone'
The calcium hydroxide does not dissolve in the acetone' actually it is quite insoluble' the mole we are seeking does' 5 O-DMT'
Decant the acetone and evap it'
Of what is left is a sticky brown goo'
Vapourise 20-30mg of this' 5 O-DMT'
Calcium Bufotenine does not really exist'
only in the process of the side chain reaction that forces out the H' of the 5HO-DMT' it only exists in solution or as of when in physical contact with calcium hydroxide' as a compound'~ Seems to jibe well with: Quote:“I mixed 10 grams of pure bufotenine freebase with 10 grams of pure calcium hydroxide in just enough 50% alcohol to make it easily mixed by the electric stirrer. This was stirred continuously for 12 hours. I evaporated the alcohol completely. I dissolved the resulting powder in 99% alcohol. The alcohol absorbed roughly 50% of the material. It left behind pure calcium hydroxide. The test seemed to be a failure.
I tested 10 mg of the bufotenine which should be bufotenine freebase because the reaction of forming calcium bufotenate seemed to have failed and I wanted to see for sure that the bufotenine was simple freebase bufotenine. So, I vaporized it, and the taste was very different. It was sort of soapy tasting. After about 5 minutes it produced euphoria and some light visuals effects. It wasn’t anything like bufotenine. The effects were too mild and consisted of mostly euphoria and little else. I thought maybe it got partially decomposed and was just weaker because of it.
The following day I vaporized 30 mg of this material in one large inhalation. Pure bufotenine freebase tastes a little like bit like very mild coffee to me. The taste was again soapy and not like bufotenine. The smoke was milder also. After I exhaled, I immediately started feeling a strange prickling sensation in the back of my head. Within seconds it traveled down to the rest of my body light a bunch of tiny needles jabbing me lightly. I was a bit startled by the prickling sensation. I get this from bufotenine for the minute or so, but never this strong. About 1 minute after that, the prickling sensation was replaced by a euphoric tingling sensation as I started experiencing visuals of such intensity that I cannot compare it to anything else. Everything in the room was vibrating and changing color, but the room still looked the same. After about 2 minutes colors were flying all around the room, like colorful fireworks, but the room still looked the same only it was vibrating and constantly changing color. My body felt relaxed. There were no side effects, not even slight nausea. After about 5 minutes the visual effects were really strong. With my eyes closed I saw all sorts of visions. These were actual visions of places, not just a bunch of visual patterns. The experience peaked in about 10 minutes and lasted at least 2 hours. The visuals with the eyes opened or closed were much stronger than any other psychedelic I’ve tested (bufotenine, DMT, 5-MeO-DMT, LSD, psilocin, salvinorin A) and were very unique. The euphoria was also stronger than I’ve felt from any other psychedelic. These were the only effects felts other than the prickling that quickly changed to a tingling sensation in the body. It wasn’t mentally psychedelic at all. There were no effects on the mind. There was no sense of disorientation, no clouding of thoughts. There were just very strong visual effects and euphoria. This was psychedelically inactive on a mental level, but visually more powerful than any other hallucinogenic alkaloid I’ve tried. It also did not seem to have the auditory hallucinogenic effects that bufotenine has.
After many other tests I came to the conclusion that this alkaloid is no longer bufotenine. And it can’t be calcium bufotenate either because all of the calcium was recovered after the reaction took place.
I believe the 5-HO-DMT (bufotenine) reacted with the calcium hydroxide, probably forming Ca + -5-O-DMT (calcium bufotenate), but then after drying the calcium went back to form calcium hydroxide and left behind 5-O-DMT. With the missing H at the 5 position the molecule is highly reactive just waiting for something to react with. The negatively charged O needed something to bond to. With nothing available I think the positively charged N at the end of the amine side change of -5-O-DMT became attracted to the negatively charged O at the 5 position forming a ring like alkaloid. This is the only plausible explanation.” I know that there's been difficulty in confirming the account that you posted, so I figured this made for some nice corroborating evidence. Not that I get the impression the first post comes from a very chemically analytical perspective, but the fact that the calcium was not in the altered non-bufotenine is what's important.
|
|
|

DMT-Nexus member
Posts: 5826 Joined: 09-Jun-2008 Last visit: 08-Sep-2010 Location: USA
|
SWIM knows that test was flawed. SWIM has tried many times to repeat that test and has failed since then with inconsistent results. In all of SWIM's recent attempts SWIM gets something that is inactive by vaporization at up to 30 mg, but he hasn’t tried it as a snuff yet. SWIM thinks the process is usually producing dehydrobufotenine, and that dehydrobufotenine is either inactive or simply cannot be vaporized. In the few old cases where the test produced a compound active by vaporization, SWIM believes the bufotenine was either mis-identified, or contaminated with maybe DMT N-Oxide, and that accounted for the amazing affects by vaporization. However, that test does show that bufotenine transforms into something else when exposed to calcium hydroxide at pH 12.4 for several hours. After that process, all the calcium hydroxide can be recovered, and the alkaloid can also usually be recovered separately by dissolving it in alcohol. It would be nice if someone can validate that the alkaloid being created by reacting bufotenine with calcium hydroxide is actually dehydrobufotenine. One thing that is weird is that sometimes the reaction produces a result that is insoluble in alcohol, and maybe that’s actually calcium bufotenate and not dehydrobufotenine? SWIM is totally puzzled by that. Maybe this has something to do with the length of reaction time, the amount of water, etc. SWIM just doesn’t know. SWIM keeps getting inconsistent results and he’s really not happy with it. If there is someone out there that knows more about this reaction, please help out SWIM. He’s dying to know what’s going on here. You may remember me as 69Ron. I was suspended years ago for selling bunk products under false pretenses. I try to sneak back from time to time under different names, but unfortunately, the moderators of the DMT-Nexus are infinitely smarter than I am.
If you see me at the waterpark, please say hello. I'll be the delusional 50 something in the American flag Speedo, oiling up his monster guns while responding to imaginary requests for selfies from invisible teenage girls.
|
|
|

DMT-Nexus member
Posts: 5826 Joined: 09-Jun-2008 Last visit: 08-Sep-2010 Location: USA
|
I should mention then when extraction psilocin or bufotenine DO NOT USE HYDROXIDES. Use carbonates instead. Psilocin (4-HO-DMT) is a very similar molecule to bufotenine (5-OH-DMT). SWIM’s tests show that bufotenine is destroyed by hydroxides if the pH is 11 or higher. But sodium carbonate is not capable of destroying bufotenine even at pH 11.4. A while back SWIM did some tests with bufotenine and put bufotenine in water at pH 11.0 (using an accurate freshly calibrated pH meter to check the pH) in 3 different solution: one of ammonium hydroxide, one of calcium hydroxide, and one of sodium carbonate. After 24 hours the calcium hydroxide and ammonium hydroxides destroyed all the bufotenine, while the sodium carbonate did NOT. I’m absolutely sure the same will be true with psilocin. The only difference between bufotenine and psilocin is that psilocin has a hydroxyl group on the 4 position and bufotenine has the same hydroxyl group on the 5 position. That’s it. The rest of the two molecules are identical. As stated above, it’s believed that the decomposition product of bufotenine from exposure to alkaline hydroxides at pH 11 and up is dehydrobufotenine. It’s very likely that psilocin forms a similar decomposition product, but instead of leaving the hydroxyl group the tail forms a bond with it (after the hydrogen is removed). Anyone else know what this likely decomposition product might be called? You may remember me as 69Ron. I was suspended years ago for selling bunk products under false pretenses. I try to sneak back from time to time under different names, but unfortunately, the moderators of the DMT-Nexus are infinitely smarter than I am.
If you see me at the waterpark, please say hello. I'll be the delusional 50 something in the American flag Speedo, oiling up his monster guns while responding to imaginary requests for selfies from invisible teenage girls.
|
|
|

Kalt und Heiß, Schwarz und Rot, Kürper und Geist, Liebe und Chaos
 
Posts: 4661 Joined: 02-Jun-2008 Last visit: 30-Apr-2022
|
Interesting. If this is true, then one should get a 7-position ring that also includes a N-O bond, as it happens with isoxazolidines. Ibotenic acid contains a very similar arrangement. It would be also interesting to look further on the carbonates vs hydroxide bases. Ammonia, sodium hydroxide, potassium hydroxide and calcium hydroxide provide hydroxides by reacting with the water. In contrast, in the case of sodium carbonate it is the carbonate ion that react with the water to generate hydroxides. Even though it is difficult to see the difference (the result is basically the same; OH- is enriched in the water) this does not mean that there is no difference. Need to calculate between salts and freebases? Click here! Need to calculate freebase or salt percentage at a given pH? Click here!
|
|
|

tryptamine photographer
Posts: 760 Joined: 01-Jul-2008 Last visit: 14-Jan-2025
|
Yes, eventually it's the OH- ions in the carbonate solution that make it basic... or rather the H+ / OH- equilibrium, so it can't be just the OH-. (???)
Maybe the ammonia reacts in its own way because of the NH3? It's not such a strong base, is it? Puzzling.
I wonder if this drastic 3-dimensional rearrangement of the atoms makes the DHB inactive. Maybe it ruins the shape of the molecule. That would be a pity...
Fascinating subject 69ron, thanks!
I hope to extract my first tiny bit of bufotenine soon, but maybe I have the wrong seeds... we'll see.
|
|
|

DMT-Nexus member
Posts: 5826 Joined: 09-Jun-2008 Last visit: 08-Sep-2010 Location: USA
|
I’d really like someone to explain why ammonium hydroxide can destroy bufotenine but sodium carbonate can’t. They are about equal in base strength. Both can get a solution to pH 11.4. Harmalol is soluble in ammonium hydroxide but insoluble in sodium carbonate solutions. That’s a known fact. Can anyone explain why that is? I’m sure the same principles relate to bufotenine to a certain extent since both are phenolic alkaloids. You may remember me as 69Ron. I was suspended years ago for selling bunk products under false pretenses. I try to sneak back from time to time under different names, but unfortunately, the moderators of the DMT-Nexus are infinitely smarter than I am.
If you see me at the waterpark, please say hello. I'll be the delusional 50 something in the American flag Speedo, oiling up his monster guns while responding to imaginary requests for selfies from invisible teenage girls.
|
|
|

Kalt und Heiß, Schwarz und Rot, Kürper und Geist, Liebe und Chaos
 
Posts: 4661 Joined: 02-Jun-2008 Last visit: 30-Apr-2022
|
69ron wrote:I’d really like someone to explain why ammonium hydroxide can destroy bufotenine but sodium carbonate can’t. They are about equal in base strength. Both can get a solution to pH 11.4. Technically speaking there is not such a thing as ammonium hydroxide; It is ammonia reacting with water to produce hydroxides; Quote:NH3 + H2O <-> NH4+ + OH- And sodium carbonate provides OH- by this mechanism; Na2CO3 -> 2Na+ + CO3--, then it is the carbonate ion that reacts with water in this manner: Quote:CO3-- + H2O <-> HCO3- + OH- and to a much less (and possibly insignificant) degree Quote:HCO3- + H2O <-> H2CO3 + OH- The result is the same, hydroxides are produced. But in the case of sodium carbonate, the solution is possibly getting slightly buffered. There are many more ions around other than say Na+ and OH- or Ca++ and OH- or NH4+ and OH-. Sodium carbonate carbonate dissolution gives Na+, CO3--, HCO3-, H2CO3 and OH- all together. Maybe the "protective effect lies in this ionic balance? 69ron wrote:Harmalol is soluble in ammonium hydroxide but insoluble in sodium carbonate solutions. That’s a known fact. Can anyone explain why that is? I’m sure the same principles relate to bufotenine to a certain extent since both are phenolic alkaloids. Do you have a reference for that? In any case, if that was true (not doubting!) then harmalol would be insoluble in other bases that work like sodium carbonate, like sodium citrate, sodium acetate, sodium fumarate (basically any salt that is product of any strong base and any weak acid) etc. Need to calculate between salts and freebases? Click here! Need to calculate freebase or salt percentage at a given pH? Click here!
|
|
|

DMT-Nexus member
  
Posts: 3555 Joined: 13-Mar-2008 Last visit: 07-Jul-2024 Location: not here
|
SWIMs imaginary friend has some extra bufotenin laying around and would be willing to analyze (by MS) it after making whatever it is this degradation product is.
What are the conditions that SWIY has used to generate both active and non active products?
|
|
|

DMT-Nexus member
Posts: 5826 Joined: 09-Jun-2008 Last visit: 08-Sep-2010 Location: USA
|
There are many books the state that harmalol is soluble in hydroxides and not carbonates. One such book in the Merck Index. So it is definitely a FACT. And according to SWIM's tests this also seems to apply to bufotenine to a certain degree although it’s not documented anywhere. Now the Merck Index says it’s soluble in hydroxides but not carbonates. It doesn’t mention ammonium hydroxide or any other base by name. However, another book I have does mention that it’s soluble in ammonium hydroxide and insoluble in sodium carbonate. It’s stated as a fact. I can give that reference too if you like. I don’t want to debate this fact with harmalol because it’s pointless. What I’d like to know is WHY this is the case because I don't really understand the reason for it. You may remember me as 69Ron. I was suspended years ago for selling bunk products under false pretenses. I try to sneak back from time to time under different names, but unfortunately, the moderators of the DMT-Nexus are infinitely smarter than I am.
If you see me at the waterpark, please say hello. I'll be the delusional 50 something in the American flag Speedo, oiling up his monster guns while responding to imaginary requests for selfies from invisible teenage girls.
|
|
|

DMT-Nexus member
Posts: 5826 Joined: 09-Jun-2008 Last visit: 08-Sep-2010 Location: USA
|
burnt wrote:SWIM has some extra bufotenin laying around and would be willing to analyze (by MS) it after making whatever it is this degradation product is.
What are the conditions that SWIY has used to generate both active and non active products? VERY COOL!!!!! Mix 1 gram of calcium hydroxide with 1 gram of freebase bufotenine and 50 ml of water with 50 ml of alcohol (IPA works) for 24 hours. Filter off the calcium hydroxide. Evaporate, and the product is inactive. You may remember me as 69Ron. I was suspended years ago for selling bunk products under false pretenses. I try to sneak back from time to time under different names, but unfortunately, the moderators of the DMT-Nexus are infinitely smarter than I am.
If you see me at the waterpark, please say hello. I'll be the delusional 50 something in the American flag Speedo, oiling up his monster guns while responding to imaginary requests for selfies from invisible teenage girls.
|
|
|
DMT-Nexus member
Posts: 119 Joined: 02-Nov-2008 Last visit: 29-Jun-2011
|
69ron, have you tried snorting/sublingualing the degradation product?
|
|
|

DMT-Nexus member
Posts: 5826 Joined: 09-Jun-2008 Last visit: 08-Sep-2010 Location: USA
|
No. SWIM wants to try it sublingually or by snorting. Maybe it's active by those routes. It is not smokeable at all, and sort of tastes like soapy DMT. You may remember me as 69Ron. I was suspended years ago for selling bunk products under false pretenses. I try to sneak back from time to time under different names, but unfortunately, the moderators of the DMT-Nexus are infinitely smarter than I am.
If you see me at the waterpark, please say hello. I'll be the delusional 50 something in the American flag Speedo, oiling up his monster guns while responding to imaginary requests for selfies from invisible teenage girls.
|
|
|

DMT-Nexus member
  
Posts: 3555 Joined: 13-Mar-2008 Last visit: 07-Jul-2024 Location: not here
|
SWIM doesn't have calcium hydroxide any replacement for doing this reaction? Would any base work or have differences been noticed depending on the base?
|
|
|

DMT-Nexus member
Posts: 5826 Joined: 09-Jun-2008 Last visit: 08-Sep-2010 Location: USA
|
I know calcium hydroxide works for sure. Sodium carbonate doesn't work. Ammonium hydroxide seemed to do the job. SWIM never tried sodium hydroxide, but that should work too. Calcium hydroxide is the easiest to use. You just mix the calcium hydroxide and the bufotenine together in equal amounts with 50% IPA for 24 hours. Then you filter off the calcium hydroxide. Most of it easily filters off. You then evaporate it to get your "dehydrobufotenine" or whatever it is. It's very easy to check the outcome. If it's soluble in acetone afterwards, it wasn't converted, it's still bufotenine. Bufotenine is soluble in acetone, but the "dehydrobufotenine" or whatever it is, is insoluble in acetone. You may remember me as 69Ron. I was suspended years ago for selling bunk products under false pretenses. I try to sneak back from time to time under different names, but unfortunately, the moderators of the DMT-Nexus are infinitely smarter than I am.
If you see me at the waterpark, please say hello. I'll be the delusional 50 something in the American flag Speedo, oiling up his monster guns while responding to imaginary requests for selfies from invisible teenage girls.
|
|
|

John
Posts: 700 Joined: 31-Aug-2008 Last visit: 27-Jan-2024 Location: Highland
|
burnt wrote:SWIM doesn't have calcium hydroxide any replacement for doing this reaction? Would any base work or have differences been noticed depending on the base? Have about magnesium hydroxide bro? Can one use Mg(OH)2 or not? As a kemist I never met ILPT in physical form and never talk to him. He share his wisdom, trough my mind, telepathicly only. Please don`t prosecute me, for his possible illegal activities. He is bonkers about chemistry and doesn`t even exist in this primitive reality !!!
|
|
|

Kalt und Heiß, Schwarz und Rot, Kürper und Geist, Liebe und Chaos
 
Posts: 4661 Joined: 02-Jun-2008 Last visit: 30-Apr-2022
|
69ron wrote:Calcium hydroxide is the easiest to use. You just mix the calcium hydroxide and the bufotenine together in equal amounts with 50% IPA for 24 hours. Then you filter off the calcium hydroxide. Most of it easily filters off. You then evaporate it to get your "dehydrobufotenine" or whatever it is. Is the addition of IPA necessary for the conversion? Wouldn't just water do? Need to calculate between salts and freebases? Click here! Need to calculate freebase or salt percentage at a given pH? Click here!
|
|
|

DMT-Nexus member
Posts: 5826 Joined: 09-Jun-2008 Last visit: 08-Sep-2010 Location: USA
|
SWIM always used 50% IPA for his tests because it makes the calcium hydroxide much less soluble in the water, making it easier to filter out at the end. Water alone should work. You may remember me as 69Ron. I was suspended years ago for selling bunk products under false pretenses. I try to sneak back from time to time under different names, but unfortunately, the moderators of the DMT-Nexus are infinitely smarter than I am.
If you see me at the waterpark, please say hello. I'll be the delusional 50 something in the American flag Speedo, oiling up his monster guns while responding to imaginary requests for selfies from invisible teenage girls.
|
|
|

DMT-Nexus member
  
Posts: 3555 Joined: 13-Mar-2008 Last visit: 07-Jul-2024 Location: not here
|
SWIMs instrument is temporarily busted. Will be a while before SWIM can do analysis. Stuff happens.
|
|
|

DMT-Nexus member
Posts: 5826 Joined: 09-Jun-2008 Last visit: 08-Sep-2010 Location: USA
|
That sucks...I really want to see this tested out. SWIM would do it if he had the equipment, but he doesn't and can’t expense anything like that right now. He has too many bills to take care of at the moment. You may remember me as 69Ron. I was suspended years ago for selling bunk products under false pretenses. I try to sneak back from time to time under different names, but unfortunately, the moderators of the DMT-Nexus are infinitely smarter than I am.
If you see me at the waterpark, please say hello. I'll be the delusional 50 something in the American flag Speedo, oiling up his monster guns while responding to imaginary requests for selfies from invisible teenage girls.
|