Ok all pictures back online now again.
I also reuploaded the prediction of Yuremamine at the first page.
And that day I said *Ah well I dont see how Yuremamine could be hidden inside "
3.) 1H+13C NMR Spectra of *Jungle Spice*",
but now I actually watched twice on it and I maaaay got an interesting insight on this.
In the following there is a slice of the predicted spectra and the one from
3.). And I think the Signals between the aliphatic and the aromatic range (4 - 5,5 ppm) somehow look similar. They have the same multiplicity and roughly the same shift. Also there are no other signals within that range, so IF Yuremamine would be hidden in this spectrum, these signals could indeed be those.
Even the integral is correct: it's a
1:3 based on the one with further shift. Hmmm suspicious.
Here is the picture of the comparison:
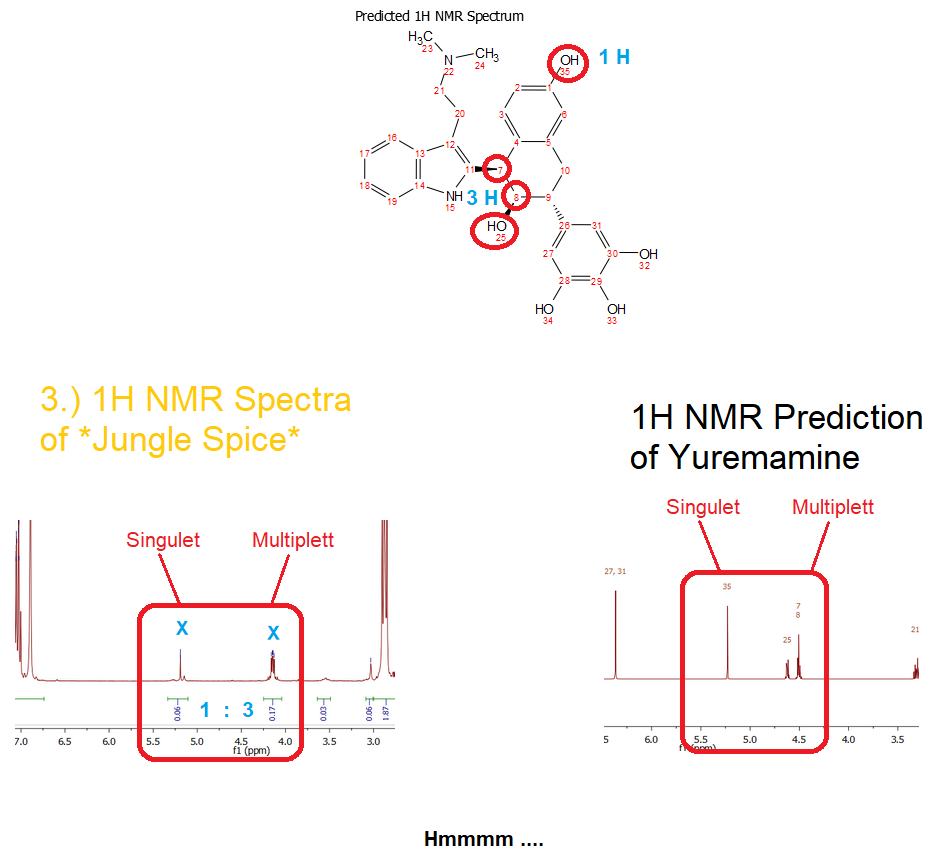
Ok but how about other signals that would then also be in the spectrum of
3.)? (referring now to the numbers given by Mestrenova 12.0 at the predicted spectrum at page 1 and in the picture above)
Where is the big
Dimethyl-Signal 23 + 24?
-> it could be the one at
2,08 ppm - The shape of this signals even looks very much like a strong methyl signal
Where are the
aliphatic signals 9, 10, 20, 21 in the 2,6 - 3,3 ppm range?
-> they could be
hidden below all those other big peaks in that range. They would just be minor artefacts - not that safe, but it could be. As the prediction is not 100 % accurate, they may indeed be hidden, even though the prediction may place some peaks outside of the measured ones.
Where are the
aromatic signals 27, 31, 2, 6, 3, 17, 18, 19, 16 in the 6,5 - 8 ppm range?
--> they are definetly
hidden below all those other aromatic peaks in that range. As the prediction is not 100 % accurate, they may indeed be hidden, even though the prediction may place some peaks outside of the measured ones.
Where are the
phenolic protons 32, 33, 34 ?
--> they would not appear, as they are even more
prone to D-H-exchange than regular alcohols. Therefore we observe proton 25 as it is not a phenolic alcohol, but not the ones above. Ok but then it is
strange that phenolic proton 35 still would have been measured ... this is a contradiction to the stuff just told.
Ok so now there are only the signals at ~ 4,5 - 5,25 ppm left that should be visible definetly if Yuremamine is hidden in this spectrum - and there are some signals like those indeed in
3.).
And now why are there none of those in the 13C-NMR Spectrum?
C-NMR is way less sensitive so it may not catch up all the minor components, therefore it would not be too surprising if we dont observe any signals of Yuremamine there. Nevertheless there are a lot more aromatic signals and also (obviously) at different chemical shifts, so these still could be the Signals from that other aromatic rings in trace amounts.
Not sure if this is a tiny proof, but at least I would not totally exclude the presence of Yuremamine in
3.) anymore. Sadly I did not do a MS of that sample that days.
So Yuremamine was originally extracted using a cold methanol soak and was thought to be destroyed upon heat. It's a big molecule so that is very likely, but has there actually been a proof of this? I think nobody ever outside "serious research" had pure Yuremamine and tested what happened in aqueous solutions at 70 °C +.
Just a brief addition:
I looked for the XLogP3, which gives an idea about the polarity and therefore the solubility. DMT is at 2,5 and Yuremamine at 3,1, which would indicate that
Yuremamine should be
less polar than DMT. That is strange, because it seems with Naphtha Yuremamine is not extracted,
only with stronger solvents like Toluene or Methanol.
downwardsfromzero wrote:It is very likely worth taking a look at the predicted spectra for 2-methyltetrahydro-β-carboline (IMO)...
I completely forgot about what you wrote that time ago, maybe gonna add this soon and look for signs if the Tryptoline is in there. I read it's possible that under high heat tryptamines may form their tryptoline forms. But I forgot the name of that reaction ;o