DMT-Nexus member
Posts: 8 Joined: 01-Jan-2013 Last visit: 21-Jan-2021 Location: UK
|
Blimey, you put a lot of work in don't you endlessness!
Kudos.
I'm not a Chemist or botanist, but reading through this thread, a thought occured to me.
While gramine is undesirable, could it be that when naturally contained within a plant, Garmine has a synergistic relationship with DMT?
In other words...could it be possible that plants containing a relatively high % of Garmine, also contain a proportionate % of DMT too?
I wonder if it is easier to test for the presence of Garmine within a plant, and use that as an indicator for the presence of DMT? And should we be looking at Garmine containing plants with a view to using that chemical as a basis for possibly identifying DMT content?
Probably talking rubbish, but if you don't ask...
Cheers.
|
|
|
|
|
member for the trees
  
Posts: 4003 Joined: 28-Jun-2011 Last visit: 27-May-2024
|
spikey wrote: Quote:While gramine is undesirable, could it be that when naturally contained within a plant, Garmine has a synergistic relationship with DMT? ..Gramine in animal studies was only toxic at very high doses compared to what would be present in a theoretical extraction..also, Gramine has had use as a human health supplement.. but there are known non-Gramine strains (Big Medicine, AQ1 etc) so it shouldn't be an issue except for the field-researcher..
|
|
|

DMT-Nexus member
Posts: 23 Joined: 02-Apr-2008 Last visit: 07-Oct-2014 Location: tasplace...
|
Exellant work neverend! Great thread interesting stuff im currently doing studies with some local phalaris verieties il post back soon with any findings. Also i read somewhere that garamines melting temp was 100c more than dmt and if vaped carfully doesnt leave apperatus, thats if thats all you where to do with it. Peace stay true to self , things will follow through enter decay
|
|
|

Explorer, Creative and Curious
Posts: 925 Joined: 08-Jan-2012 Last visit: 04-Dec-2015 Location: West Coast of Canada
|
I think what spikey is saying... Look for garmine in other plants you didn't know had dmt and you might be suprised to find there is a relationship between garmine and dmt present. Thus a gold mine of dmt hidden in many plants that we never thought could have dmt. Look for garmine and find a hidden dmt treat! Sorry if this is not what you meant, but I think you did and it must have zipped over their heads! Cheers! Done: THC - LSD - MESC - MDMA - Shrooms - DMT / Want:Hyperspace travel - World Peace
Respect, intention, meditation, inhalation, observation, analyzation, respect.
|
|
|
DMT-Nexus member
Posts: 18 Joined: 04-Feb-2013 Last visit: 08-May-2015 Location: USA
|
Pleasantly surprised today from a GC-MS analysis of a methanol extraction of some oven-dried phalaris brachystachys. I heard talk of "up to 3% DMT", but I was sure it was only hype. I don't have a "calibration standard" for DMT but compared to some MHRB crystals, it could very well be 3% (of dried weight). The other good news was that the gramine peak was only about 1/20th or less of the DMT peak. However... it seemed to have a closer ion profile to 5-MeO-DMT than N,N-DMT. Perhaps the two substances co-elute with the capillary column being used. Also, the extraction was of young (three week old) phalaris brachystachys, and perhaps the profile favors N,N-DMT as the plant ages (just guessing).
At any rate, it appears to be a perfect candidate for Ayahuasca; plus no bitter tannins.
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 22-Nov-2025 Location: Jungle
|
Thanks for sharing. Was it wild harvested or grown from seed? Any more info you can share on the plant, conditions of growth, climate, etc? Can you share the GC-MS specs ? Maybe its possible to get the raw data file so we can take a look at it? 5-MeO-DMT should be easily differentiated from DMT. Look at the mass spec of both.. 5-MeO-DMT : 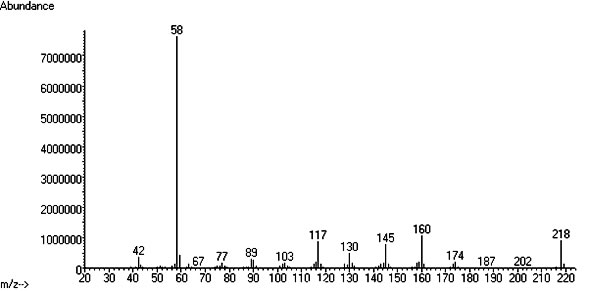 and DMT 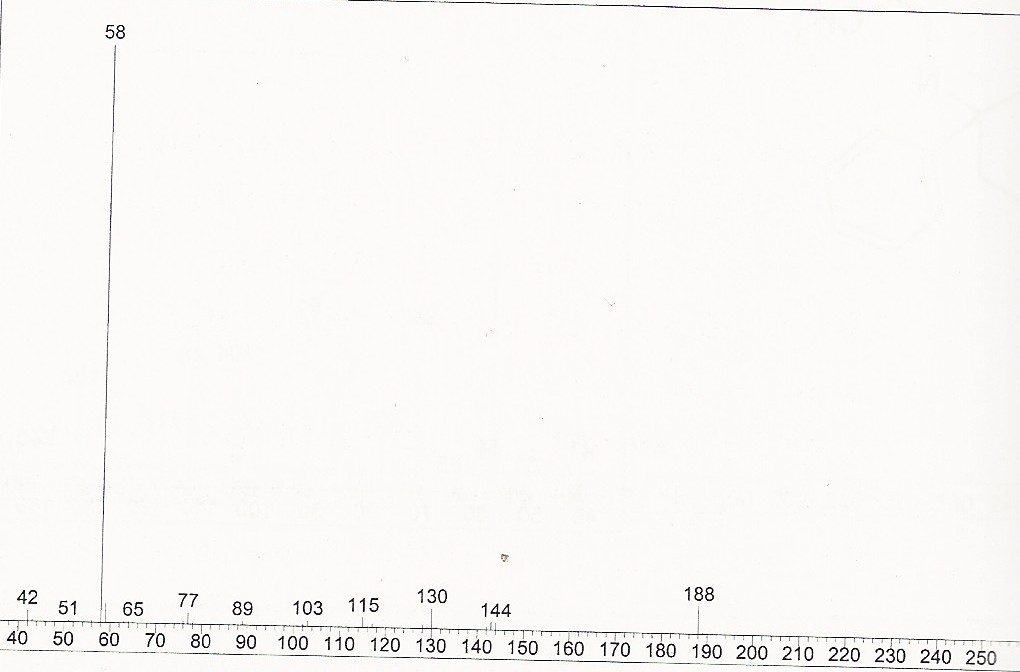 So do you see the 188 ion in your supposed dmt peak? what about the 160 and the 218 one? btw regarding the question earlier regarding gramine vs DMT, there is some publication out there that mentions there is an inverse relationship between gramine vs dmt but we ve analysed a couple of plants that had both, so I dont think its such an easy division... Before we know more on gramine's effects on humans, Id rather steer in the side of caution and avoid plants containing it, or rather, extracting those plants and separating the wanted alkaloids from unknown ones, before consuming it.
|
|
|
DMT-Nexus member
Posts: 18 Joined: 04-Feb-2013 Last visit: 08-May-2015 Location: USA
|
Endlessness... Is it possible that NN-DMT is converted to 5-MeO-DMT when it is extracted in methanol by sonication?? I hear the plants were grown from seed bought on the internet, so until the plants get seed-heads it is difficult to know for sure if they are phalaris brachystachys . Here is a (poor) photo. I should mention, the spectral image is from the analysis referred to in my earlier post. LivingInAwe attached the following image(s):  PhalarisB0001.jpg (145kb) downloaded 997 time(s). PIC_0289.jpg (417kb) downloaded 991 time(s).
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 22-Nov-2025 Location: Jungle
|
Thanks again! I am not a chemist, but I don´t think the DMT will turn into 5-MeO-DMT... BUT, notice as quoted in the first post in this thread, in Trout´s SST, Justin Case mentions that enzymatic activity may affect alkaloid content after harvesting, but they mention that to favour 5-MeO-DMT content, one needs to dry the plants before processing them. So if you got 5-MeO-DMT after harvesting and processing without drying, then thats probably what your plant had in the first place.
And yeah, that spectra is most definitely 5-MeO-DMT, NOT DMT.... Was it the main peak? Do you have a scan or picture of the TIC with the other peaks?
Is the vendor listed in our seed vendors subforum? If not, you might want to share with us because a plant high in 5-MeO-DMT is an interesting thing for us to know.
Any more info you have to share, feel free! Thanks again!
|
|
|
DMT-Nexus member
Posts: 18 Joined: 04-Feb-2013 Last visit: 08-May-2015 Location: USA
|
Endlessness...
I was told the Phalaris brachystachys was harvested and immediately oven dried at 100 deg C for about one hour. The chromatograph was looked at carefully in that region for a tryptamine peak other than 5-MeO-DMT, but was not to be found. NN-DMT (no longer available)from Mimosa Hostilis was analyzed on the same instrument about a two months ago, but with a different column. The retention time for that analysis compares very closely to the RT for 5-MeO-DMT on the new column, when the retention time shift differences are applied (11.03 minutes for NN-DMT on old capillary column vs 12.27 minutes for 5-MeO-DMT on the new column). Soon my friend will have enough plant material to do a A/B extraction and will perhaps be able to verify this strange finding. Also, I did not see the seed vendor listed, but I was not able to post his ebay name because I am a new member.
|
|
|

DMT-Nexus member
 
Posts: 12340 Joined: 12-Nov-2008 Last visit: 02-Apr-2023 Location: pacific
|
I dont think it is brachystachys in that picture. It looks like it is branching in some spots which I never saw my brachys do. I could be wrong though. I think brachys just shoots up one single leaf blade per seed and thats it. Long live the unwoke.
|
|
|
DMT-Nexus member
Posts: 18 Joined: 04-Feb-2013 Last visit: 08-May-2015 Location: USA
|
Jamie, Did you let it go to seed to verify the species? The only picture I could find on the internet which displayed the base of the Phalaris brachystachys is this one: http://davesgarden.com/g...es/pf/showimage/186646/
A scientific discription of the phalaris brachystachys can be found at http://www.kew.org/data/...ses-db/www/imp07942.htm
(caespitose: A caespitose or cushion plant is a plant with short stems and branches that grows in dense tufts or clumps, with the flowers held above the clump or tuft.) Don't get too alarmed; as Endlessness said, it may be because I am oven drying it, the enzymes are greatly favoring the 5-MeO-DMT. I thought that oven drying would be better than air drying because the enzymes wouldn't have a chance to do their job and make 5-MeO-DMT, but as most of my ideas, that assumption could be completely wrong, and the heat of the oven may just make the enzymes work faster and better. What I lack in intelligence I make up with persistence, so I will get to the bottom of this. I am very excited about the possibility of having a choice of NN-DMT or 5-MeO-DMT just by controlling the processing of the plant... How exciting is that! I will post further analysis updates within a week (I will try a methanol extraction of FRESH plant material without drying).
|
|
|

DMT-Nexus member
 
Posts: 12340 Joined: 12-Nov-2008 Last visit: 02-Apr-2023 Location: pacific
|
yes it went to seed and was brachys. Getting it to put out enough seed to not have to rely on vendors every year to restablish a useable patch is another issue though it seems. Long live the unwoke.
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 22-Nov-2025 Location: Jungle
|
LivingInAwe wrote:Endlessness...
I was told the Phalaris brachystachys was harvested and immediately oven dried at 100 deg C for about one hour. The chromatograph was looked at carefully in that region for a tryptamine peak other than 5-MeO-DMT, but was not to be found. NN-DMT (no longer available)from Mimosa Hostilis was analyzed on the same instrument about a two months ago, but with a different column. The retention time for that analysis compares very closely to the RT for 5-MeO-DMT on the new column, when the retention time shift differences are applied (11.03 minutes for NN-DMT on old capillary column vs 12.27 minutes for 5-MeO-DMT on the new column). Soon my friend will have enough plant material to do a A/B extraction and will perhaps be able to verify this strange finding. Also, I did not see the seed vendor listed, but I was not able to post his ebay name because I am a new member. Thanks again! Regarding retention time, unless you tested the specific substance in the specific column, its very hard to make generalizations. Even if one substance was at similar retention time in one column, on another column they may be at very different retention times. Also even if the peaks overlaped, we would probably see some of the characteristic ions in the mass spec there (for example 188 for DMT). So indeed it seems just 5-MeO-DMT. As for testing fresh vs dried, remember to have control groups when doing the tests, so for example mix/homogeneize a bigger amount, then divide that in two, and half you dry, the other half you fresh extract, and then compare, because if you do it in different times/days or with different parts of the plant, as you probably know the comparison will not be valid. Im not sure if changing between DMT and 5-MeO-DMT in a plant is as simple as changing the harvesting/drying, I would guess there are some inherent characteristics/limitations based on genetics, and that you will only able to turn the balance to one side till a certain extent. But Im just thinking out loud here, the only way to really know is to do what you are doing, to test and analyse 
|
|
|
DMT-Nexus member
Posts: 18 Joined: 04-Feb-2013 Last visit: 08-May-2015 Location: USA
|
Endlessness... My friend did what you suggested: Collected an assortment of Phalaris Brachystachys clippings from a variety of plants in various locations. Diced up the clippings and mixed well, then divided the pile into two piles. One pile was oven dried (as before) and the other was frozen and then methanol extracted. The two extracts (Dried vs. essentially fresh) analyzed by capillary column on GCMS were virtually identical; both chromatograms showing mostly 5-MeO-DMT, with just trace amounts of N,N-DMT (The N,N-DMT was not originally noticed in an earlier analysis). I would appreciate any thoughts/ideas of why these findings seem to conflict with other research, suggesting that the main alkaloid in Phalaris Brachystachys is N,N-DMT. It DOES seem to be quite high in 5-MeO-DMT though. LivingInAwe attached the following image(s):  FDPB02220001.jpg (202kb) downloaded 920 time(s).
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 22-Nov-2025 Location: Jungle
|
Excellent work!
So freezing seems to stop enzymatic reactions from happening. It´s possible that if it was left for long without processing (drying, freezing, etc) it would be different.
Im wondering about yields of this plant too. Please if you do any extract, dont forget to let us know about extraction method, what yield is, etc. Also any further analysis is of great interest, we might see (or not) a variability even using the same seed, so that would be interesting to know. If you want, you can write now in the suppliers section part regarding the vendor.
|
|
|

DMT-Nexus member
Posts: 1453 Joined: 05-Apr-2009 Last visit: 02-Feb-2014 Location: hypospace
|
If this is not appropriate for this thread I do not mind it being moved: Suspected Phalaris paradoxa (paradoxa grass) poisoning in horses Aust Vet J 2003;81:635-637 Quote:Herbage samples from five accessions of
P paradoxa, two of P coerulescens and one of P aquatica
(unknown cultivar), were soaked in dilute acid (0.1 m mol / L
HCl) for 16 h. The extracts obtained were treated with solid
phase cation exchange resins and analysed using TLC and
HPLC as previously described...
The major alkaloidal components (AKL Note: this is not for aquatica, just for paradoxa and coerulescens)
were phalarine, coerulescine and 2-methyltetrahydro-b-
carboline (2Me-THBC). In contrast to the other samples, the
relative amount of 2Me-THBC in the sample CPI 14073
(Italian heritage) was predominant, with only trace amounts of
phalarine and coerulescine.
Also trivial but fun from same article: Quote:Horses for example have the ability
to detoxify 5-methoxy dimethyltryptamine and convert it into
the less active and more rapidly excretable compound bufotenine
(5-hydroxy dimethyltryptamine). This has been demonstated
in horses in the United States by administering 5-
methoxy dimethyltryptamine orally and then finding bufotenine
but no 5-methoxy dimethyltryptamine in post treatment
urine samples (RA Sams et al, personal communication). It has
also been indirectly demonstrated in Australia by the finding
that horses grazing P aquatica pastures during late winter and
early spring can excrete bufotenine in their urine If I had seeds of CPI 14073... would there be an interest in growing it out and testing it? 
|
|
|

DMT-Nexus member
Posts: 336 Joined: 01-Jul-2011 Last visit: 29-Jun-2024 Location: Gaia
|
very interesting paper about the enzymatic bio-pathway of creating dmt in phalaris aquatica. it talks alot about the enzymatic processes which take inside the plant. im interested if it explains something on the enzymatic processes which take place when the plant is drying. here is the paper.. http://www.plantphysiol....ntent/88/2/315.full.pdf
Quote:This paper reports the activities of enzymes and concentrations of reactants of the DMT synthesizing pathway as a function of age of P.aquatica seedlings for the first 16d of seedling growth. These data together with the kinetic constants of the enzymes have been used to model the DMT synthesizing system mathe- matically during this initial 16d of seedling growth.
|
|
|
DMT-Nexus member
Posts: 57 Joined: 03-Jun-2013 Last visit: 24-Sep-2013
|
I read that paper quite some time ago. For some reason I favored a single INMT enzyme hypothesis. I guess because they didn't chromatograph and purify the proteins. They do say that the 2ndary INMT (SIMase) acts on both NMT and 5MeO-NMT. So both DMT and 5MeO could increase after harvest. Perhaps the kinetics favor one over the other in certain conditions. Interesting you posted that article... Quote:...genetic evidence in P. arundinacea indicates that gramine synthesis is independent of DMT' and 5MeODMT formation (11). This is further supported by the finding that the N-methyltransferase activities involved in gramine synthesis are different from those involved in the synthesis of DMT and 5MeODMT. Seems to disfavor any gramine/DMT association, but that should already be fairly apparent from the structures and pathway. I was pretty jazzed to see this discussion going strong! I reviewed that paper just prior to seeing your post about it. I've recently begun an experiment on P. brachystachys, and have just started cultivating 10 varieties from across it's natural range. I'd like to hear if anyone has any good ideas on simple assays for gramine and other alkaloids. I had assumed I'd do quantitative TLC on them and visualize on fluorescent plates and/or with p-DMAB. But seeing some of the techniques here, I'm wondering if there isn't a way to do smaller and faster qualitative assays that I could do in larger numbers. I'm planning to start a breeding program if there seems like enough variation in the germplasm to be worth it. Any ideas would be appreciated. -FF
|
|
|

DMT-Nexus member
Posts: 8 Joined: 30-May-2013 Last visit: 03-Sep-2013
|
deleted “Coincidence is God's way of remaining anonymous.”
― Albert Einstein
|
|
|
DMT-Nexus member
Posts: 57 Joined: 03-Jun-2013 Last visit: 24-Sep-2013
|
So I've got 7 varieties of P. brachystachys growing. They are from Jordan, South Africa, Greece, Libya, Morocco, Israel, and Algeria.
I've been thinking on how to analyze them on the group and individual level.
So far my thought is to take a plant clipping, run it through a rolling mill to extract the juice. I'll take a standard aliquot of the juice and add it to a test tube of acidic polar/non-polar solvent. Shake well, and take off the polar layer, concentrate under vacuum to a set amount, then run on a TLC plate and develop with p-DMAB.
Seems to me that there could be plenty of issues with this process, but I'm trying to figure out a way to quickly analyze lots of samples for my breeding program.
A few questions...
How much material will I need for the TLC? I'm assuming a minimum of around 25ng to visualize, and thought maybe 50-100ng would be a good amount to try. But I have no idea how much liquid material this would be since everything is usually given in dry weight.
Is a simple acidic polar/NP extraction/wash going to result in pure enough starting material, or am I going to end up with a bunch of bio-gunk that will just form a smear on the TLC?
I can certainly run 7 TLCs using more standard methods, but if I want to really analyze individual plants for a breeding program that number will get pretty large pretty quickly. If I can figure out a good method for quick analysis that would really help things along.
I'm also looking for some cites on gramine TLC if anyone has any handy. I recall reading a paper doing 2D TLC looking for gramine, but if anyone knows some good cites I'd really appreciate it since I can't seem to find the paper again.
-FF
|