
Faustian Phytochem Investigator
 
Posts: 194 Joined: 31-Oct-2011 Last visit: 29-Nov-2025 Location: Oaxaca
|
endlessness wrote:Doz, check out the TLC plate on the top of this last page. Notice line 7, which corresponds to this wash that we're talking about. Do you notice on top, in that streak of fumaric acid? Well if you look well, appart from the fumaric acid, there is a distinct clear spot there, that shines on UV. Im wondering if thats the same substance youve been getting, with a higher Rf than DMT.. I think you could be right that the top spot in lane 7 of your plate might well be 2MeTHBC. I'm fairly certain that spot 1 in plate 20 is not 2MeTHBC. It doesn't fluoresce, and it shows up as blue with xanthydrol. 2MeTHBC will show as pinkish purple in xanthydrol as it doesn't have any higher numbered substitutions. However, spot #2 in lanes C1 and C2 which does fluoresce and shows up as pinkish purple is a very likely candidate. Looking at plate 20 again, I would also suspect that spot directly below the DMT spot is DMT oxide. It shows a similar rF when I looked at the oxide plate. That extraction had quite a bit of manipulation over the course of a week or more, so it wouldn't be too surprising. As for T-1 and T-2, I suspect these are degradation products. What is it that makes you suspect that they are tryptamines? Not that I disagree, just curious as to why. I wouldn't be surprised if these are in relation to Alk 274 from the paper. My suspicion is that there is the possibility that yuremamine breaks down into these heavier compounds that keep the indole portion and also to the phenol complexes that you are finding with lower weights. I'm thinking that it might even break down to different compounds depending on what's been done to it analytical wise... It's just a hunch, but one that I've had for a very long time. It's kind of what drew me to the Nexus in some ways.
|
|
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 22-Nov-2025 Location: Jungle
|
Dozuki wrote:
I think you could be right that the top spot in lane 7 of your plate might well be 2MeTHBC. I'm fairly certain that spot 1 in plate 20 is not 2MeTHBC. It doesn't fluoresce, and it shows up as blue with xanthydrol. 2MeTHBC will show as pinkish purple in xanthydrol as it doesn't have any higher numbered substitutions. However, spot #2 in lanes C1 and C2 which does fluoresce and shows up as pinkish purple is a very likely candidate.
Looking at plate 20 again, I would also suspect that spot directly below the DMT spot is DMT oxide. It shows a similar rF when I looked at the oxide plate. That extraction had quite a bit of manipulation over the course of a week or more, so it wouldn't be too surprising.
As for T-1 and T-2, I suspect these are degradation products. What is it that makes you suspect that they are tryptamines? Not that I disagree, just curious as to why. I wouldn't be surprised if these are in relation to Alk 274 from the paper. My suspicion is that there is the possibility that yuremamine breaks down into these heavier compounds that keep the indole portion and also to the phenol complexes that you are finding with lower weights. I'm thinking that it might even break down to different compounds depending on what's been done to it analytical wise... It's just a hunch, but one that I've had for a very long time. It's kind of what drew me to the Nexus in some ways.
Thanks Dozuki! Do you write down the Rf's of the spots? Regarding T1 and T2, my naming may have been kinda misleading. T-1 I really think its a tryptamine compound because: 1- NIST gives it a 72% chance of being N-Acetyltryptamine (the main difference is that the base peak with T1 is 130, and second highest is 143, and with N-Acetyl-t, its the inverse... T-1 also seems to have molecular weight of 202 just like N-acetyl-t. I know mass spectra can change a bit, even with pure substances, but Im not sure if so much that the base peak itself changes and inverts with the second largest peak... they are pretty close in terms of abundance, maybe in those cases its possible they invert?) 2- All the other guesses from NIST, though with smaller chance, are tryptamine/indole-based compounds (4-(1H-Indol-3-yl)butanamide , : N-β-Chloropropionyltryptamine, 2-(1H-Indol-3-yl)ethyl acetate, etc ). I guess the base/second peak are characteristic of some of these 3- T-1 was also found in small amounts in Acacia acuminata extract from nen/ I guess this is not enough for an identification, specially given my lack of experience with analytical chemistry but it was enough for me to have a hint it was a tryptamine/indole substance. Here's the mass spectra of T1 vs N-acetyl-T 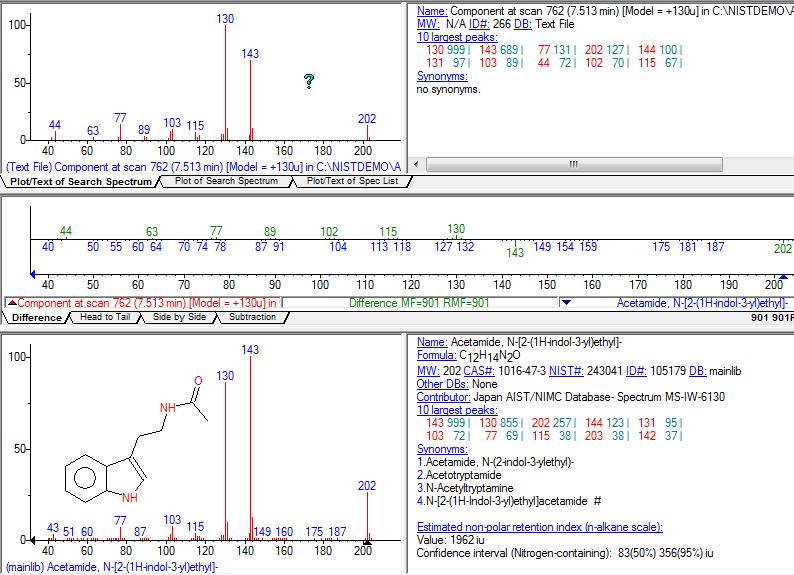 T2 I have no idea if its a tryptamine or not, mass spectra is nothing like ive seen, and best NIST guess is 6% chance, not very good to have an idea... Dozuki, whats your take on the phenetylamines, arent you surprised by that too? Lastly, does anybody here thinks (some of) the information in this thread can be turned into a publication, or is it all too amateur?
|
|
|
member for the trees
  
Posts: 4003 Joined: 28-Jun-2011 Last visit: 27-May-2024
|
..i think some of this great work you've done for the nexus could be semi-formally or formally published, but peer review and some help eliciting the unknown compounds would help..
..it seems to me that M. hostilis is actually quite similar to many acacias in chemistry, with similar trace compounds..
.
|
|
|

Faustian Phytochem Investigator
 
Posts: 194 Joined: 31-Oct-2011 Last visit: 29-Nov-2025 Location: Oaxaca
|
endlessness wrote:Thanks Dozuki! Do you write down the Rf's of the spots? I do occasionally, but I mostly just compare my plates and the relative positions of the compounds. I have images of the plates and can calculate the rF values from the images easy enough. I don't use analytical grade solvents so my rF values are going to be a little different than the literature. For example I use 30% AcOH as opposed to 99% as that is what I have. Comparative positions and color reactions to UV, and spray reagents help to further indicate possible substances. I just received some silicotungstic acid, so I will soon add that to the repertoire. And I wan't to start with UV-Viz here soon. Quote:Dozuki, whats your take on the phenetylamines, arent you surprised by that too? Not really. Hordenine, which is only one hydroxy group away from N,N-dimethyl-phenethylamine is found in Mimosa ophthalmocentra. Again, I would suspect these are degradation products and link back into tannins. My thinking here is that tannins complex with DMT in the plant to produce yuremamine (for the plants and the plant parts that produce it). Under extraction conditions yuremamine breaks down into degradation products that resemble the tannins and tryptamines. I suspect what it breaks down to is highly variable upon the extraction conditions. The phenethylamines could be one of these YDPs. The N-methyl-phenethylamine MS looks like a good match, however, the 2nd one seems 'off'. This would seem to indicate the possibility of being a weird degradation product. There are other spectra in all of these analyses that show the same base peak (185 for instance) but then have different spectra such as T-2 and some of the suspected beta-carbolines. They have some similarities (m/z 185, 144, 113-114) but then have totally different M+. Seems like it might be one compound breaking down in various different ways. I spent the evening drawing variations on what the break down products of yuremamine might be and figuring out the molecular weight. I keep going back to the quote in the original 2005 yuremamine paper: "Unidentifiable degradation products". So far I haven't been able to match anything up precisely. Inside the molecule of yuremamine, you can see N-methyl-penethylamine, and N,N-dimethyl-phenethylamine, DMT, NMT, and phenol based compounds related to the tannins. It's all in there, it just seems to be a big cosmic puzzle as to how it comes out. Realize however, that this is still my working *hypothesis*. It was borne of an 'AH HA!' moment that I had when reading that original 2005 paper after being highly puzzled over the disappearing spot #1. I thought that the NMT spot was one of the degradation products as well. At that time the consensus thought was that MHRB was a very pure source for DMT. There were really only a couple papers on it (known), one of which claimed it was the sole alkaloid (via paper chromatography). The work being done here is showing compounds like NMT and the beta-carbolines in MHRB where that has not really been show before. I think some of these other compounds are being produced by the plant, but we have to sift through what is a natural product of MHRB and what are degradation products, and analysis artifacts (DMT oxide for example). I think there is publishable data here. How to go about it is another thing. What publication? Above ground or underground publication? For any above ground scientific journals you might have to compare your results with reference samples. Also, the identification of the starting material might have to be more precise. Getting root bark from a middle man vendor, its impossible to know the true identity of the species being looked at is. With the work for Acacias, this might be a little easier as you could obtain a specimen. MHRB specimen might be another story, I don't know. I think that its awesome that results are getting published here on the Nexus! That in itself is publication. The only reason that I found The Nexus was because Entropymancer used some of my plates in the paper he got published in The Entheogen review. I discovered that via Google one day because I was curious what ever became of the whole yuremamine/jungle spice mystery and I read his drafts of that paper here. Either way, this is ground breaking stuff that I feel privileged to be involved with.
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 22-Nov-2025 Location: Jungle
|
Good feedback again! I think its interesting regarding the degradation product theory. I think the way to test it would be when we get a LC-MS, hopefully now in february, and then run some mimosa and see if there is any sign of these phenetylamines, appart from yuremamine...
Im not experienced with column work, but that could be another way to attempt isolating these phenetylamines from mimosa without destroying yuremamine, if they are not simply degradation of it..
Im not sure what else we could do to determine whether something is degradation product /analysis artifacts... I guess LC-MS would really help is settle this.
Regarding botanical identification, that is a good point. I dont know how we could make it reliable? Just asking for more info from vendor, like detailed pictures and all, and getting a botanist to look at it wouldnt be enough I guess?
Regarding the publication, soon we should get some reference samples which could help with the publication too. Any further ideas what coud be done?
|
|
|

Faustian Phytochem Investigator
 
Posts: 194 Joined: 31-Oct-2011 Last visit: 29-Nov-2025 Location: Oaxaca
|
Further evidence of yuremamine degradation products: After spending another night drawing molecules (instead of drawing half naked women at a 'Dr. Sketchy's' sketching night) I have come to some understanding of what we are seeing in the Mass Spectra of MHRB. I will try to explain the best I can and will refer to the attached images of the sketches. First, what is actually in MHRB as I can tell from the data in this thread it contains: NMT DMT 2MeTHBC possibly some Phenols and Pyrogallols I believe pretty firmly that the rest of the peaks are yuremamine degradation products (YDP) including the suspected beta-carbolines 1,2-DMTHBC and MTHBC. They appear to be beta-carbollines because the yurmamine is breaking down into 3 closed ring compounds that are very similar to beta-carbolines once the pyrogallic and phenolic rings 'A' and 'E' are strip off of the molecule. See YDP 'C', 'H', and 'F' in the second drawing. These 2 stripped rings show up in the MeOH soak as catechol, resorcinol, and homocatechol due to the heat of the GC-MS and possibly even due to the heat of room temp. extraction stripping them off the yuremamine molecule (Vepsalainen 2005 extracted at 5 C.). In regards to 1,2DMTHBC; the M+ 200 is YDP 'H', the base 185 peak is the resulting breakdown of the YDP to a structure resembling 'C', then 'F', further breaking down possibly to the 128 peak that resembles 'E' with de-protonation of two hydrogens in the MS. The sequence of this example as the MS breaks it apart is: A - B - H - C - F- E and then further smaller peaks. A similar sequence of ionization in the MS can be seen for the other compounds that were thought to be 1,2-DMTHBC such as in the Unwashed Jungle Spice MS. The interesting thing about those other peaks is that they also show smaller (in abundance) fragments at much higher m/z such as 351. So, technically the M+ of these YDPs should be M+351, M+398, etc. Other clues to this in outside research are one of Gardner's (2011) tryptamine compounds with an M+201 which could possibly be a protonated 'H' YDP and Radio879's 2006 MS of a xylene extraction shows a peak with an M+350 as well. As for the MTHBC, as endlessness suspected, this is not in MHRB but is another YDP. The first drawing shows Gutsche and Harderich (1997)'s explanation of why MTHBC has an M+186 and a base 158 via the Retro-Deils-Alder reaction in the MS when studying beta-carbolines. The data here shows M+ 186 base 171, a different spectra, tho similar. Because this YDP is a 3 ring structure similar to the beta-carbolines, it will have somewhat similar peaks, but the overall peak 'fingerprint' will be different. In the data shown here, I believe that what was thought to be MTHBC at first is actually the compound labeled T-2 as it shows peaks of 185, 144, and 128. The sequence of ionization starts from an M+285 and follows: A - B - C - D - E with de-protonation of 3 hydrogens of E (Ignoring the higher and lower m/z fragments). Important peaks for YDPs seem to be 186, 200, 171, 144, and 130 as they all share two or more of these peaks +/- 3 hydrogens. I tried to outline some of the ionization paths in the drawing and that is what the arrows are. I'm sure more of the peaks could be sussed out, but I feel like these are enough to start to show what is going on. Now yuremamine just needs isolated again to show that the compound is actually in there. It's the ghost in the machine since it is such an unstable molecule. Dozuki attached the following image(s):  MTHBC.jpg (160kb) downloaded 414 time(s). YDP Peaks.jpg (902kb) downloaded 421 time(s).
|
|
|

Faustian Phytochem Investigator
 
Posts: 194 Joined: 31-Oct-2011 Last visit: 29-Nov-2025 Location: Oaxaca
|
And here's the drawing re-done on the computer for clarity. These represent the peaks in the MS and not the actual YDP. The YDPs most probably have M+ between 477 and 200. Dozuki attached the following image(s):  Yprog.jpg (147kb) downloaded 333 time(s).
|
|
|

Faustian Phytochem Investigator
 
Posts: 194 Joined: 31-Oct-2011 Last visit: 29-Nov-2025 Location: Oaxaca
|
Here are a couple more examples. They are the suspected 1,2DMTHBC and the T-2 Peak from the last posted MS results. They show the probable fragments in the MS Peak as they relate to YDPs. Dozuki attached the following image(s):  t-2peak.jpg (369kb) downloaded 362 time(s). 2cDMTHBexp.jpg (316kb) downloaded 342 time(s).
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 22-Nov-2025 Location: Jungle
|
Amazing contribution Dozuki! Thanks a lot for all the work and thinking along!
The substance which I suspected would be 1,2-dimethylTHBC, and that you gave another possibility (You called H), would have IUPAC name 2-(2,3-dihydro-1H-pyrrolo[1,2-a]indol-9-yl)ethanamine
But you drew something wrong, the amine with that N is not possible, dont you mean NH2 ? Or am I missing something. But yeah it would have the molecular weight of 200.13 and the fragments would match what we see in the mass spec... Good work!
But what about T-2, whats your proposed structure for it?
Ill come back to this thread soon, really good development there dozuki, thanks!
BTW, I edited the thread to include your very educated hypothesis its a YDP, and also I just noticed what I first thought was psilocin contamination in FASW, is actually clearly dmt n-oxide, which would of course make sense.
|
|
|

Faustian Phytochem Investigator
 
Posts: 194 Joined: 31-Oct-2011 Last visit: 29-Nov-2025 Location: Oaxaca
|
You are correct, the computer drawing is missing the 2 hydrogens, they are in my hand drawing. I will correct the computer drawing when I get the chance. I haven't gotten around to figuring out any of the potential fragments higher than 200 yet, but will see what I can work out. Again, I have a feeling that yuremamine will break down to different fragments depending on the extraction/analysis conditions.
|
|
|
member for the trees
  
Posts: 4003 Joined: 28-Jun-2011 Last visit: 27-May-2024
|
..yeah great stuff Dozuki..!  , thanks, endlessness also.. re: yuremamine..is it not possible that processes of drying and aging could degrade the yuremamine into byproducts? ..if so, then fresh material may be where the 'ghost in the machine' lives.. re: botanical verification..certainly the most rigorous phytochemical papers involve depositing a voucher specimen of the extracted plant with a botanical gardens..this is possible with the acacias..perhaps possible with first-hand mimosa suppliers..full leaf material for clear ID should suffice.. ..there's also the ongoing conjecture as to whether the brazilian vs. mexican forms of M. hostilis are chemically identical, and whether or not other Mimosa species are substituted or confused with it.. lastly, given your i think spot on theory on the degradation products of yuremamine, Dozuki, i think the results on acacia acuminata are interesting, and, as i said in the Complex Indole Alkaloids thread, there may be more of these 'hyper-molecules' on other live plants, which degrade into multiple compounds upon usual extraction/analysis methods.. .
|
|
|

Faustian Phytochem Investigator
 
Posts: 194 Joined: 31-Oct-2011 Last visit: 29-Nov-2025 Location: Oaxaca
|
Okay, Here are a couple more possible fragments. I like the 351 fragment. Seems pretty stable and is only 1 hydrogen off from the 350.1 in Radio879's MS and the Unwashed Jungle MS @ 6.211 minutes has a peak at 351, but I need to look at that peak again in Open Chrom. The other image is of a *possible* fragment for peak 285. AMDIS actually shows this @ 284 if you zoom in. So, weight is right. However, I can only get this weight by opening up the benzene rings. I know those rings are pretty stable so this bugs me a little bit. Also, the AMDIS program doesn't register them. But I also realize that its not always picking up other peaks like DMT N-Oxide and such. So, those peaks might be noise, or it might be that the GC-MS is breaking open those two benzene rings. Either way, the rest of the peaks weigh out pretty well against all the other fragments that I drew up the other day for the M+ 285 (M+284). nen888 wrote:re: botanical verification..certainly the most rigorous phytochemical papers involve depositing a voucher specimen of the extracted plant with a botanical gardens..this is possible with the acacias..perhaps possible with first-hand mimosa suppliers..full leaf material for clear ID should suffice.. Ideally one would want a stem with leaves and flowers for proper ID. I hadn't thought of contacting one the direct suppliers in Mexico or South America. That might be a way to do it as they do have websites and e-mail. Dozuki attached the following image(s):  351.2.png (29kb) downloaded 326 time(s). untitled.png (20kb) downloaded 326 time(s).
|
|
|
member for the trees
  
Posts: 4003 Joined: 28-Jun-2011 Last visit: 27-May-2024
|
..certainly the flowers (and pods) would be required for a formal botanical confirmation, however this may be a tall order for the MHRB suppliers in the near future..also, it is important to test plants while they are not in flower, as in the case of some acacias, things are very changed..i figure a leaf sample of the mimosa can allow 1) fine magnified observation of hairs and glands; 2) possible future DNA thumbprinting (as this is being done to most chemically interesting plants worldwide now) . edit: actually, maybe the DNA won't preserve..let's just find some nexians growing m. hostilis.. 
|
|
|

Faustian Phytochem Investigator
 
Posts: 194 Joined: 31-Oct-2011 Last visit: 29-Nov-2025 Location: Oaxaca
|
Looking at the unwashed jungle spice sample again, it looks like what I thought was a 351 peak is definitely noise as it doesn't show up in mMass. But this does give some more possible examples for Radio 879's MS. @endlessness - can you point me to NMT in any of these mimosa samples? I'm really not seeing it. I've looked on the front side of the DMT Peak, but there really isn't anything there that I can see. And also where is it in the Acacia samples for comparison? A few more, one of which is from Entropymancer's document: Dozuki attached the following image(s):  350-1-1.gif (18kb) downloaded 312 time(s). 284.gif (16kb) downloaded 312 time(s). 284-2.gif (16kb) downloaded 313 time(s). 266.gif (17kb) downloaded 311 time(s).
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 22-Nov-2025 Location: Jungle
|
Thanks dozuki! Indeed it seems the mass weight of T-2 is 284, the higher peaks are noise. Pretty weird though, if we have to open the benzene rings there, hard to understand whats going on... Can we think of any other possibility appart from YDP? Regarding N-Oxides, they do appear in GC-MS, except part of it seems to convert back to the parent compound. In this paper, they say: Quote:approximately one third of clozapine-N-oxide was determined to be reduced to clozapine under the described capillary GC conditions. On-column reductions of N-oxides to the corresponding tertiary amines find a precedent in the property of other tricyclic drug N-oxides subjected to the generally more reactive conditions of packed column GC [12-14]. (...) thermal degradation of this Noxide to the parent drug may result in an overestimation of clozapine concentrations Now interestingly, when i tried to make an n-oxide with 10% hydrogen peroxide, it did seem to create the dmt n-oxide peak (eluted at around 5.7, with the mass peak at 204, in the file im attaching, same as with FASW file eluted at around 5.72). But if we take the above paper to be right also for DMT, then the n-oxide amount is probably being under-estimated because some of it is converting back to the parent compound. LC-MS will help settling this. The other interesting thing is that the supposed N-Oxide product I created has several other peaks appart from dmt n-oxide and DMT.. It also has a very large quantity of 2MTHBC and several other components.. In the paper mentioned above, when analyzing the pure n-oxide, they also came up with 3 peaks instead of one.. Here's what they said: Quote:Formation of these latter components may represent thermally induced Meisenhiemer rearrangement [15] (C-N oxygen insertion) and/or enamine forming processes [13,14], both characteristic GC degradation pathways of N-oxides. I wonder how much of this explanation is valid for the other compounds we're seeing in the n-oxide analysis, and how much are actually other degradation products of DMT .. ? Even in the TLC of the n-oxide there were other peaks appearing, so maybe its not only in the gc-ms but DMT can oxidize to other things with hydrogen peroxide, it seems (maybe also with air.. ?) Here's the TLC plate:  Maybe 3 = 2MTHBC ? ? Now take into account what infundibulum said to me by pm (hope you dont mind me sharing, inf) : Quote:This is basic rule in chemistry and oxidation is such a generic term and used to describe an array of diverse reaction and products. Believe it or not, the cyclisation of dmt to 2MTHBC is actually an oxidation reaction! Burning dmt is also an oxidation reaction, an extreme one; all organic substances burn (or oxidise) to CO2 and H2O. So an oxidation product of dmt is carbon dioxide and water, also some nitrogenous compounds due to the presence of nitrogen as well. But there are so many other oxidation intermediates and by-products, like 2MTHBC as mentioned I also googled "indole oxidation" just to get a rough idea of what else can happen to dmt and damn, it is diverse! one can get indole fusion by products (like in here: http://www2.iq.usp.br/docente/lhc/lab/indole.pdf) or just addition of oxygen atoms such as here: http://en.wikipedia.org/...dole_NBS_Oxidation.png. And psilocin also is thought to be oxidising to a blue compound, maybe of similar structure to indigo (http://en.wikipedia.org/wiki/File:Indigo.svg). So when one employs H2O2 he's sure to oxidise the tertiary amine of dmt to n-oxide, but he may be oxidising the molecule in who knows in how many other different ways. We may also need to take into account that some oxidations may be fast (like the oxidation of n to n-oxide) but others may be slower. Once I tried to make some n-oxide using peracetic acid and yes, it turned deep red pretty quickly (maybe n-oxide?). I left it alone for few days and the oxidation appeared to be proceeding and the deep red started changing to clearer yellow (maybe hell knows further oxidised products?) And with air oxidation, who really knows what may be happening; some n-oxide formation? some other oxidation by products? Still all too interesting and crying to be tested! How interesting, that 2mthbc amount is increasing a lot when doing this oxidation test with hydrogen peroxide, as inf said, its also an oxidation reaction.... But wow, can it really be? Lastly, regarding your question of NMT, you can see NMT in the Yellow FASI sample, there is a small slope just before the DMT peak.. it doesnt even detect it as a peak but if you click on it with openchrom, you can see the mass spectra of NMT, with the characteristic 44 base ion and the 130 and 131 peaks). Its also there in acacia confusa samples, the mass spec I posted in post 16 of acacia analysis thread, together with other acacias in the zip file. So im attaching the paper ive been quoting above, as well as the supposed n-oxide gc-ms
|
|
|
DMT-Nexus member
Posts: 2240 Joined: 20-Oct-2009 Last visit: 17-Aug-2025 Location: PNW SWWA
|
I would like to graciuosly thank everyone who have beeen working on this prject, specifically, endlessness and Dozuki. I am hoping that eventually someone my be able to shine somee light on something for me. What does this really mean to me? Someone please feel free to correct my simple way of thinking here. Ice House wrote: Sooooooooo, If I get some half way decent MHRB and I do a simple STB on it and I pull 3-4 times and Re-X, I imagine I will achieve a final product in the 96% purity range. Now if I load up 35 mg of that in my trusty GVG Mission accomplished! I love the study that you guys are doing, I am attempting to follow it. I'm lost. I know there are other elements in my spice, I'm sure there are. The question is, am I getting any psychactive effects from the extra kibbles and bits in there. I kinda feel like The DMT just drowns or overwhelms everything else in the miz. Throw a dog a bone you two! I want to keeep following this thread. I'm sorry I'm such a rock. lol Ice House is an alter ego. The threads, postings, replys, statements, stories, and private messages made by Ice House are 100% unadulterated Bull Shit. Every aspect of the Username Ice House is pure fiction. Any likeness to SWIM or any real person is purely coincidental. The creator of Ice House does not condone or participate in any illicit activity what so ever. The makebelieve character known as Ice House is owned and operated by SWIM and should not be used without SWIM's expressed written consent.
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 22-Nov-2025 Location: Jungle
|
Good question Ice House! Thanks for posting your words here  And no problem with the difficulty, even I find it hard to follow the thread here, so much information and new concepts try to make some sense  My hypothesis is that these small amount of other alkaloids in typical mimosa extractions do NOT affect one's experience. They are present in so tiny amounts that I think they will not be noticeable. There are examples where even inactive substances affect the pharmacology of other active substances, but I think this would more likely be so in the case of, say, certain acacia trees where there are very high amounts of phenetylamines, tryptamines and beta carbolines. For mimosa's minor alkaloids, I think only if you really concentrated them (not even jungle spice with naphtha pulls because theres still too much dmt), then you might feel something. Nevertheless, this is important research for general plant chemistry curiosity, and if for some reason one of these other minor alkaloids proved very interesting, we could potentially make use of the knowledge here to use it.
|
|
|

Faustian Phytochem Investigator
 
Posts: 194 Joined: 31-Oct-2011 Last visit: 29-Nov-2025 Location: Oaxaca
|
I agree, that is a good question IceHouse. One of the biggest things to be gained from this is that the Fumaric acid precipitation method and the freeze precipitation method both seem to be good ways of achieving purity. So, Yes, I would agree with your quote: "Mission accomplished" Delving deeper into it, and this thread is starting to go way off into the deep end, there are some question that are trying to be answered. Like: What is 'Jungle spice' or Jungle alkaloids? What is up with yuremamine and the YDPs? What about DMT N-Oxide? Do these affect the experience? Why are there reports of MHRB being orally active without a MAOI? Other things: NMT has now been shown to be in the extractions by GC-MS. I think this has some direct bearing on nen888's NMT thread2MeTHBC has also been shown to be in the extraction by GC-MS. As far as I know this is only the 2nd finding of this compound in M. hostilis, and probably the first in the root bark. Stay tuned, who knows what the future will bring! P.S. I have some preliminary analysis on the DMT-Oxide conversion, but I'm going to start a new thread so as not to bog this one down any more.
|
|
|

Faustian Phytochem Investigator
 
Posts: 194 Joined: 31-Oct-2011 Last visit: 29-Nov-2025 Location: Oaxaca
|
Not much info out there on these other than this paper and the ref in it: Camargo-Ricalde, S. L. (2000), Descripción, distribución, anatomía, composición química y usos de Mimosa tenuiflora (Fabaceae-Mimosoideae) en México Revista Biologica Tropical, 48, 939-954. If I break these down in Chem Draw I get fragments of: 210 208 194 190 188 174 162 148 134 120 106 Kukulkan A and Kukulkan B, the plumed serpents: Dozuki attached the following image(s):  KULKA copy.jpg (49kb) downloaded 260 time(s). KUKULKANB copy.jpg (50kb) downloaded 259 time(s).
|
|
|
member for the trees
  
Posts: 4003 Joined: 28-Jun-2011 Last visit: 27-May-2024
|
..lots to ponder! great.. Dozuki wrote: Quote:NMT has now been shown to be in the extractions by GC-MS. I think this has some direct bearing on nen888's NMT thread ..actually, i was going to add a postscript to the summary of my lecture in that thread, which was to say that, due to the rapid darkening of NMT, i am open to the possibility that 2MeTHBC is contributing to the effects..the best chemical advice i can get is that the NMT is probably breaking down into this, or 1,2,3,4-tetrahydro-beta-carboline..it must be degrading to something to explain the color change, which was also observed by Shulgin...
|