1- Colorimetric testsOverviewColorimetric are qualitative tests to help telling presence or absence of a substance. They are liquid reagents that change to different colors depending on the substance.
There are different kinds of colorimetric reagents which are more appropriate depending on which compound you are testing. Some of the most used are: Marquis, Mecke, Mandelin, Ehrlich/Van Urk/P-DMAB.
To test, you only need a tiny sample of the substance (such as, say, 1mg), and add a drop of the reagent, which will turn into a specific color.
Composition and preparation of reagents:Please take care of safety precautions when preparing and handling these reagents. Wear protective clothing, gloves and goggles. Detailed info on composition, preparation and safety data of many of these reagents in
this publication (edit: Dead link, trying to find original doc)
Test results:To see to which color do different alkaloids turn with colorimetric tests, check
this thread.
Limitations:The limitations of colorimetric relate to when a substance has a mixture of compounds. When testing with marquis, for example, a mixture of MDMA with some other substance such as amphetamine, the reaction will favor the MDMA side and therefore turn black, instead of showing the orange for amphetamine presence. Also, testing with just one reagent might give a misleading result (for example with Marquis, DXM and MDMA can look similar with a dark purple/black color), so doing a second test with another appropriate reagent (in this case Mecke, which would make DXM yellow instead) is recommended.
Colorimetric tests are good as a first screening method but for appropriate analysis one needs to use it in conjunction with something like TLC, as explained below.
Regarding safety, be careful when handling these colorimetric reagents. Marquis reagent for example is made of sulfuric acid (can cause burns) and formaldehyde (is a known carcinogen and can cause irritation of mucous membranes). Have some baking soda solution to neutralize spills in your skin.
Further links of interest:Suppliers of colorimetric reagents:
http://dancesafe.org/products/testing-kits (USA)
http://www.bunkpolice.or...earch-chemical-test-kit/ (USA)
http://eztest.com/ (europe with worldwide shipping)
http://stateofmind.nl (worldwide shipping)
http://www.chemicalgeneration.com.au/products.htm (australia - contact before, dont know if still exists)
http://ez-test.com.au/ (australia)
Reactivo de marquis (Argentina)
Possible walk-in stores to buy testing reagents in Australia and New Zealand (
Link)
Other info:
Erowid FAQ on colorimetric tests (with some color schemes)
2- TLC - Thin Layer ChromatographyOverviewTLC is a method of separating different compounds that are in a mixture.
It works in the following fashion: First of all, you dissolve a small amount of sample (say, 5mg) of the substance to be tested, in a couple ml methanol. Then you get about 3 microlitters of this solution with a capillary pipette or tube like this:

These tubes are very thin and they suck the liquid without the need of any vacuum source, simply by submerging it in the solution it will suck up a few microliters through the capillary action.
Then, one will load these 3 microlitters on the bottom of the silica TLC plate by touching with the microcapillary on the line near the bottom of the plate. Like this:

Then what one will do is, have a container with a small amount of a solvent mixture, called the Eluent. The eluent mixture depends on what substances you are testing. For tryptamines it is often some mixture of ethyl acetate and methanol, or methanol and ammonia. This all can be changed/adapted according to the solvents at hand, but some mixtures will work better than others for separating certain compounds, some will not separate at all if you use a wrong mixture.
Then one will put the silica plate standing upright in this container with the solvent mixture. The amount of eluent in the container should be small enough that only the very bottom of the silica plate is submerged in the eluent (below the place where you added the sample spots). So what happens is, the silica plate is porous and slowly soaks up the solvent. The eluent has a certain polarity, and the silica has another, so as the plate soaks up the solvent, it also carries the samples being tested upwards, and depending on the polarity of the molecules in the sample, some will travel further up, others will not travel very much upwards, so you get distinct spots.

So you basically let the plate soak up till the top part, then you remove it from the container and let it air-dry. Then you look under UV light for better seeing the spots. Usually the commercial silica TLC plates have a fluorescent indicator, which will make the spots more visible when looked under UV light.
InterpretationSo how to interpret the results?
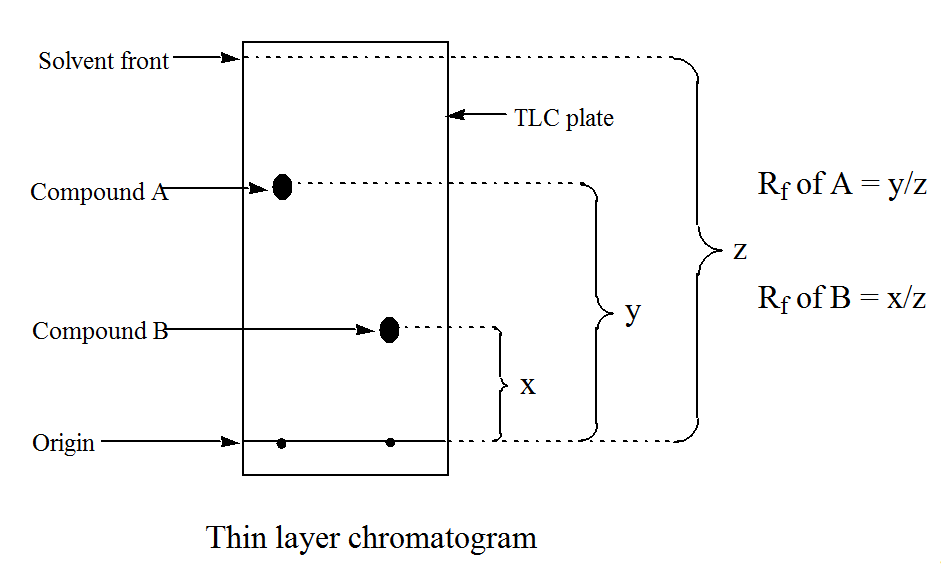
To interpret the spots there are 2 ways: Comparing with a standard that was dropped on a paralel column, or measuring Rf (retention factor) and looking at literature values to compare with your results. For measuring Rf you're gonna have to be using the exact eluent mixture that the literature value gives:
Here's how you measure Rf:
Here's an example of a plate I was working on, where I interpreted against standards and not with Rf:

Now what do we see here? There are many columns, as you see, because we were testing many things. But lets concentrate on the 3 columns on the right side with the blue spots (labelled harm, A and B).
Those are harmalas I was testing (which glow specially bright in UV light, in this case 254nm UV). Now, the left of those columns, 'harm', with the two spots, those are the standards we have. We have gotten from an official supplier, so we know they are very pure harmine and harmaline. We dropped both in the same column to "save up" on plates, because we knew they would separate neatly afterwards.
Well anyways, I wanted to test some harmine and harmaline I had supposedly separated with selective pH precipitation, as described in the
Harmala Extraction Guide. So the column labelled A was my supposed harmaline, the column labelled B was my supposed harmine. Now you see, column A has a spot which corresponds to the standard for harmaline, and very little amount of harmine on the top part. The inverse for the column B. So as we can see, my separation worked reasonably good. Even though we cant quantify accurately, at least you get an idea that there is mostly harmine on one sample, and mostly harmaline on another. If you look closely, on my harmine, there seems to be another round spot that is touching the harmine spot. It could be another rue alkaloid (THH? I had never heard of it in significant amounts in rue.. Some other harmala maybe?)
If we think of the the other non-harmala samples in the plate that we were testing, you see some have 2 strong spots in the same column (for example sample 901 and 902). This means whatever substance that was, it had a mixture. The final part of this would be to drop some colorimetric tests on each spot and see what color they turn, and compare with our standards and/or literature information, and then one can eliminate most doubts.
Limitations:Testing very crude plant preparations (such as, say, soaking some plant material in methanol and testing this methanol) will usually not work because it pulls too many impurities such as chlorophyll, plant oils and others, which end up leaving a streak that goes all the way up the plate instead of separating into distinct spots. Therefore to test with TLC you need to make at least a mini crude extraction.
Also ideally one will have the relevant standards to compare with the tested substance. If one doesnt, then it becomes a harder task because you might see, say, two different spots when measuring a material, but how do you know whats each spot? One can look at literature to see if one finds more info, drop the colorimetric tests in each spot and see if it helps showing what it is. Depending on what it being tested, its possible, but if its an unknown mixture, it will be very hard to know what is each spot.
Another aspect is that TLC is qualitative, not quantitative, at least in theory. You cannot judge, say, how much there is of one alkaloid and of another in the same mixture depending on spot size, because some alkaloids by their chemical nature dont form strong spots, etc. BUT, in theory you can make some quantification if you have pure standards of a substance. Then you would make a few columns with different known amounts of such substance (for example 100mics, 300mics, 600mics, 1mg, or whatever). Then you would put it in eluent as normal, and once you have your spots, you would take a picture/scan it, and measure the width of the spot in each amount, and then next times if you use the exact same eluent mixture, you can do a (more or less crude) quantification of that specific substance.
Further links of interest:Nexus thread: DIY TLCPost 11: TLC Rf for DMT, DMT N-Oxide, NMT and 2MTHBC
3- UV-Vis Spectrophotometry OverviewSpectrophotometry is an analytical method that can give you the purity/quantity of a certain substance. It is composed of a machine about the size of a printer, that is connected to a computer. What it does is to shine a range of frequencies from UV to visible light, through a transparent container containing a solution (usually methanol or ethanol) of the substance to be tested, in a specific proportion (often 1mg/ml). On the other side of the container there is a detector. Each substance has a unique light absorbance factor (which usually you can find in literature). So the detector will give you a graph of the light spectrum from the substance being tested, something like this (random image from internet):
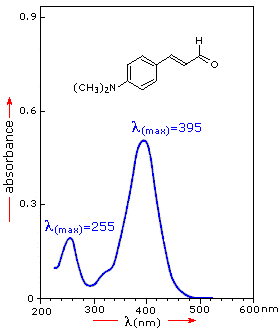
Now, with these peak numbers, you compare them with literature, and you'll get the purity/amount by doing a "rule of 3" (so for example, imagine you got a reading of 200 for your dmt testing. So if 100% dmt = 254 (just inventing the number for explanation sake), and X% = 200, so 200/245x100= 78% pure DMT. )
Limitations:Photospectrometry does not work to tell you what is inside, rather how much of a known substance is there. So before you photospec, you need to identify the substance being tested (with a mass spec or at least a TLC). Once you know which substance you are testing for and it's extinction coefficient/absorbance, you can quantify it. You could in theory identify the substance (or at least narrow down the possibilities a lot) because each substance has unique UV spectra, but only if you know you have a pure substance there to begin with, because other substances in any significant amount mixed in will change the spectra so it will be impossible to identify
4- Gas Chromatography OverviewGas Chromatography is method to separate compounds. It is usually coupled with another section, a detector, such as mass spectrometer, to identify (and quantify in some cases) the separated compounds. In the same lines as the TLC, the separation happens due to polarity difference of substances (which is related to how the electric charges are distributed in a molecule).
A solution with the substance or mixture of substances to be tested is put in the injector, and the mobile phase, which is an inert gas, like nitrogen, helium, argon or CO2, will carry it through the column, which is the stationary phase. Due to the different polarities of the mobile and the stationary phase, some compounds will pass through the column first and others later. Then the substances pass through some kind of detector, often a Mass Spectrometer, and the information will be sent to a computer to be analysed.
The gas chromatography works is like an oven, with heat, to make the molecules change to gaseous state and pass through the column. Heat might range between 30C - 320C. This will be chosen depending on what is being analysed. If one is analysing a mix of substances with a wide range of boiling point, the temperature might be programmed as a gradient over a certain amount of time. The lower the temperature, the longer it will take for a sample to pass through the column. A temperature just above the boiling point of the substance will make it take from 2min-30mins to pass through the column. Other variables which affect the speed is the flow rate of the gas, the type, diameter and lenght of column, the volatility of the compound and the polarity of the compound.
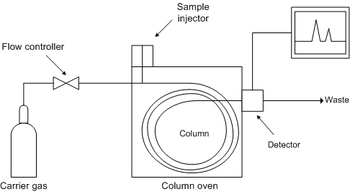 Detector
DetectorThere are several kinds of detectors, each with their own characteristics, such as Flame Ionization Detector, Thermal Conductivity Detector, Flame Photometric etc. The type of detector will usually be chosen due to the nature of the substances being tested (and the equipment available)
Commonly though, the detector used is a mass spectrometer, and the coupled instrument is abreviated as GC-MS. So after having passed through the GC, the substances will reach the Mass Spectrometer, which will have an ion source that will bombard the substances with electrons. The substances will be fragmented by these collisions, and these fragments will be detected by their mass-to-charge ratio. This information is be sent to the computer and ploted in a graph, which is the Mass Spectra (or singular, Mass Spectrum).
Interpreting data
The Mass Spectra is a graph, where the x-axis is the relative abundance (or amount of the substance), and in the the y-axis is the mass-to-charge ratio (which will tell you which substance it is). As each substance breaks appart, it will leave one or more peaks which will be plotted in that graph. This graph is compared to some database or library of published mass spectrum of compounds, or one will have a reference standard for the suspected compounds at hand to analyse and compare. If none of these options are available, the chemists or technicians can try to work out the structure of the compound by analysing the fragment patterns left by the ion collisions.
Limitations:The fact that GC-MS uses heat creates a problem for analyzing certain compounds. Some compounds will not be very volatile, and might not pass adequately through the column or might break down. Therefore one needs to derivatize these compounds with a derivatization reagent. This will slightly change the structure of the compound, still allowing it to be analysed but also making it more volatile.
5- Column Chromatography OverviewColumn chromatography is a method for separation and therefore purification of compounds. It uses similar principles than that of TLC and GC, as described above.
Column chromatography is composed of a column, packed with a stationary phase, usually made of
Silica gel or Alumina. They are basically materials with small pores, which will let the eluent phase (a solvent with the dissolved substances to be separated) pass. Due to different polarities of each substance in the mixture you want to separate/purify, a substance will elute (or in other words, pass through the column) before or after.
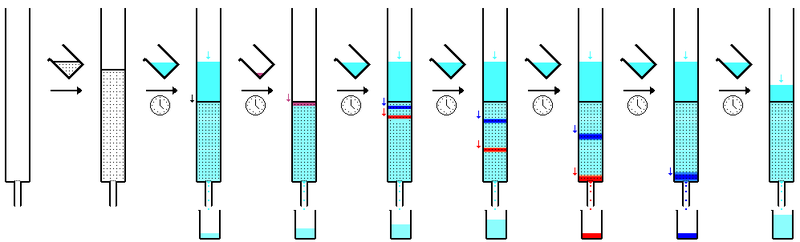
In the picture above you can see how the compound mixture is separated as the eluent carries it down the stationary phase.
Prepparing a columnThe first thing about preparing a column is choosing an appropriate sized column. If one wants to separate a few milligrams of material, a very small thin column is enough. If one is separating a gram or several grams of material, then larger columns will be necessary.
Here you see an example of 3 columns, two of them improvised and the third one on the right being specifically made for column work. The left column will be enough for 100mg or less of material. The middle column for about a gram, and the right column for many grams of material.
So once you chose your column, you typically have to add some plug or glass wool on the bottom so that when the eluent runs through, it doesnt carry away the stationary phase.
Then you must add the stationary phase. Have your column standing upright and clamped to maintain position. Then either you just add the stationary phase dry, and then later flush the eluent till the whole column is wet (and make sure it doesnt empty/dry out, till you have finished using the column), or you make a slurry with your eluent and stationary phase and then fill it up. Its important to make sure one doesnt have bubbles.

Once the column is preppared one will add a small concentrated solution of the compound mixture to the top of the column, and then open the bottom clamp just so that it starts entering the stationary phase. Then one will start adding the eluent and letting it drain out, collecting the eluent that comes out the bottom, and over some minutes the mixture should be separated.
Now, if it was a very visible colored mixture, this separation would be easier. When we're talking about colorless transparent alkaloids dissolved in the eluent, one will not know when it has been eluted or not. So either one will look at literature and past experiments to know how long it might take for such alkaloid to travel down (but this will of course depend on the lenght of column, stationary and mobile phase), or one will use this method in conjunction with a TLC. So basically every X amount of time, one will collect the eluent coming down in a separate labelled container. Then one will do a TLC test with each of these containers, to see in which containers one has the substance one desires.
Limitations:Column chromatography works to separate compounds. It is not a technique to tell you which exact compound or how much of it is in your mixture. Also as said above, for colorless dissolved substances, one will not know exactly when the substance has eluted or not, so this will depend on experience or using an exact set up that someone else has used and has published or personally communicated the time taken for elution.
Still to come: LC-MS If anything isnt clear or if you got suggestions or corrections in general, please go ahead
