
DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 22-Nov-2025 Location: Jungle
|
And another TLC plate:  Column 1 - Harmine/harmaline mixture (mostly harmaline) Column 2 - Caapi copy from FV (one can see harmine, harmaline and a spot under harmaline, which might or might not be THH) Column 3 - Supposed to be an attempt at reducing harmalas (same as column 1) with zinc to make THH, but mixture was too dilute and cant see much in the plate. Looking closely/tilting the screen, one can see a streak that seems to be on the height of the bottom spot of column 2, maybe evidence for THH? GC-MS will tell. It was an experiment done with infundibulum and the traveler here at home last weekend when they came for a visit, we used zinc, vinegar, and based with sodium carbonate (so there was a lot of excess zinc carbonate on the precipitation). Column 4 is same substance as column 1, but done the hydrogen peroxide treatment like described above for DMT.. One can see clearly that harmine is affected, very likely formation of harmine n-oxide (Which was orange/red btw) Column 5 is mimosa Column 6 is mimosa treated with hydrogen peroxide, seems it didnt change much Column 7 is a water wash of limonene after all FASI dmt has been retrieved (so shows there are still alkaloids left behind after FASI, you can see dmt and nmt spots, and the top streak is fumaric acid) Column 8 is dmt from water freebase crystalization Column 9 is dmt supposedly oxidized (the dmt spot is still there, but seems there is another spot on top of dmt, same height as the spots on top of mimosa's dmt too, maybe that is dmt n-oxide in mimosa? or something else at same height? There is a spot from column 10 that is visible, ignore that, that was another unrelated substance that I cut out of the picture
|
|
|
|
|

DMT-Nexus member
Posts: 206 Joined: 12-Jul-2010 Last visit: 15-Oct-2024
|
I cant see the picture  . Good work anyways endlessness! Do the molecules separate by density? size?
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 22-Nov-2025 Location: Jungle
|
Any of the pictures or just this last one? As a simple description, the molecules separate by polarity, which has to do with distribution of charges in the molecule. More polar molecules have a more uneven distribution of charges, and they stay closer to the bottom of the plate. Less polar molecules travel further up. You can see a description of how TLC works here: https://www.dmt-nexus.me...aspx?g=posts&t=24225I still wonder about this n-oxide plate, because its quite different than my first crude attempt at making it in the first plate on this thread... I will need to repeat this experiment again, and maybe use another eluent mixture
|
|
|

Kalt und Heiß, Schwarz und Rot, Kürper und Geist, Liebe und Chaos
 
Posts: 4661 Joined: 02-Jun-2008 Last visit: 30-Apr-2022
|
Nice job, as always! Too bad to see that the THH conversion sample was too little for detection (lane 3)...so we'll have to wait for the MS. I am also curious about lane 9, i.e. the dmt oxide one; comparing to lane 8, (just dmt) the main spot appears to have different mobility to just dmt alone. is it supposed to be the N-oxide? Could also the tinier spot on top being another oxidation/degradation product of dmt. (which as the plate suggests may also be present in mhrb)? Need to calculate between salts and freebases? Click here! Need to calculate freebase or salt percentage at a given pH? Click here!
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 22-Nov-2025 Location: Jungle
|
The thing is, that when substances are in very different concentrations, the spots may go a bit upwards or downwards in the plate... And its pretty clear that it was more concentrated in the DMT spot than in the oxide spot (I had just eyeballed the amount of DMT and methanol before adding to the plate, and the same with the methanol used to dissolve and test the n-oxide that was on the petri dish drying), so its hard to say if the oxide spot being lower is a change or if its related to concentration. I will repeat the TLC tests on thursday, also try to get a stronger concentration of the "THH" to see something (though anyways it was sent to GC-MS), and I will post here. I was wondering, can skatole be a degradation product of DMT? Would that explain the fact that it is found in mimosa and also in the supposed oxidized DMT? https://www.dmt-nexus.me...aspx?g=posts&t=28697Or maybe that higher spot is n-oxide? Or maybe they are both different substances that have similar polarities and just by coincidence are at the same height.. I curious about MS results, I think next week only though..
|
|
|

Faustian Phytochem Investigator
 
Posts: 194 Joined: 31-Oct-2011 Last visit: 29-Nov-2025 Location: Oaxaca
|
If you have 30% H2O2, you can oxidize the sample right on the plate. I read about this technique in the literature. This helps eliminate the difference in sample loading. I tried both this method and the conversion separately. I got the same results from each as far as rF of DMT and the oxide.
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 22-Nov-2025 Location: Jungle
|
Maybe 10% will work too? By the way, how did the oxide appear on your plate?
|
|
|

Faustian Phytochem Investigator
 
Posts: 194 Joined: 31-Oct-2011 Last visit: 29-Nov-2025 Location: Oaxaca
|
On 2 plates, both run in IPA:NH4OH:H2O the rFs were: DMT: .75 & .76 Oxide: .68 & .69 The slight differences in rF is probably a slight measurement error. No big deal. In the 2nd plate, the DMT sample and the oxide sample where co-chromatographed as well as run separately on the same plate. I never did get a sample to fully oxidize, they always left some un-oxidized DMT in the sample. Maybe an 80% conversion? I was using 30% H2O2, but it was a little old and not stored in the fridge and I figured this was contributing to the residual DMT. So, I would imagine 10% would work, but it probably not be a complete conversion. All the refs I have call for 30%. Aftter running these plates, I never saw any spots that would indicate the oxide in any of the other plates I ran. IE: the only time I've run into the oxide was by oxidizing the sample. I didn't think I had a picture of the plate, but turns out I photographed most of them: Spots from lowest rF to Highest: Lane #1: DMT sample: NMT - DMT Lane #2: Co spotting: NMT - DMT oxide - DMT Lane #3: Oxide sample: NMT (Slight trace) - DMT oxide - DMT The slight trace of NMT might not be visible in the image at this size, but is there when enlarged. Dozuki attached the following image(s):  Plate8.jpg (41kb) downloaded 669 time(s).
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 22-Nov-2025 Location: Jungle
|
Hmmm, so this would match more closely with what showed in the first plate in this thread, the n-oxide becomes a lower spot... I wonder what happened with this latest n-oxide then?
It clearly changed something, the smell became much sweeter, instead of the light flowery dmt smell from the 'control' sample, and it formed this yellow/orangy oil that wouldnt crystallize. When dissolved in methanol, the methanol was clearly yellow, where the original sample was white and when dissolved in methanol it just appeared transparent... But in the plate, it seems at the same height as the other DMTs (from mimosa and FASI wash), and though lower than the DMT frm control sample, as mentioned before it could be due to concentration.. So Ill have to repeat this test, see what we come up with.
Another question, anybody knows if DMT N-Oxide would show a different UV-Vis curve?
|
|
|

Faustian Phytochem Investigator
 
Posts: 194 Joined: 31-Oct-2011 Last visit: 29-Nov-2025 Location: Oaxaca
|
endlessness wrote:It clearly changed something, the smell became much sweeter, instead of the light flowery dmt smell from the 'control' sample, and it formed this yellow/orangy oil that wouldnt crystallize. I noticed the exact same thing. The smell was very 'floral and sweet'. quite pleasant actually. Sample loading might be part of it, and low conversion may also be a factor. I would imagine that the UV-Vis would be different as well, tho it might be slight. Speculating here.
|
|
|

Faustian Phytochem Investigator
 
Posts: 194 Joined: 31-Oct-2011 Last visit: 29-Nov-2025 Location: Oaxaca
|
From: "Indole bases of P. peregrina (L.) Benth. and Related Species", Fish et al 1955
UV spectral data:
....................Maximum mu
DMT ---------274--283--291
DMT oxide --274--282--290
So, not much difference there.
Fluorescence analysis data:
...............position of excitation---maximum mu emmision
DMT --------------283-----------------350
DMT oxide -------283-----------------349
The differences are probably so minimal as to be unhelpful. But, this is just one reference/
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 22-Nov-2025 Location: Jungle
|
Hmmmm, thanks for that info Dozuki! Indeed seems like it wouldnt make much difference... Would be pretty hard to see, specially because with only 1 point difference, would be very hard to identify the substance and not consider it a possible measurement accuracy error or similar...
Ill test this out again with the TLC, and otherwise once LC-MS is available we should test with that... I sent for GC-MS just in case but I think the n-oxide will appear as the parent compound..
|
|
|
member for the trees
  
Posts: 4003 Joined: 28-Jun-2011 Last visit: 27-May-2024
|
..could not DMT also partially decompose into NMT..?
also, the 2MeTHBC could also be an 'artifact' in the plant, rather than due to extraction/test method, couldn't it..?
|
|
|

Faustian Phytochem Investigator
 
Posts: 194 Joined: 31-Oct-2011 Last visit: 29-Nov-2025 Location: Oaxaca
|
I'm inclined to suspect that NMT is found in the material and not a decomposition product. It shows up in TLC plates done with a simple methanol soak, no heat or base.
As for the 2MeTHBC, really not sure.
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 22-Nov-2025 Location: Jungle
|
I just asked benz in the pictet spengler thread, what is the biosynthetic pathway to 2MTHBC in plants, if its known.
I think NMT might be an intermediate step before DMT, since if INMT works once on tryptamine, it would make NMT, and if it works on it again, it makes DMT, right?
|
|
|

Faustian Phytochem Investigator
 
Posts: 194 Joined: 31-Oct-2011 Last visit: 29-Nov-2025 Location: Oaxaca
|
Marum et al (1979), in their studies of P. arundinecea genetics proposed this path way:
tryptophan -> tryptamine -> NMT -> DMT [ <- Also proposed by Baxter and Slaytor, 1972 for P. aquatica]
then NMT -> 2MeTHBC
and they have a ? in the pathway DMT -> 2MeTHBC (See figure #1)
"Inheritance of three groups of indole alkaloids in Reed Canarygrass", Crop Science V.19, 1979, p. 539-544
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 22-Nov-2025 Location: Jungle
|
So after doing an A/B in mimosa using limonene, and salting the limo with FASI, I retrieved all the precipitated fumarates. I added more FASI, waited for a month and retrieved the last bits of fumarates that crystallized. Adding more FASI did not result in any precipitated DMT. Then, I washed the "spent" limonene with water, and evaporated the water. This yielded in a heterogeneous product that had white crystally/powdery parts, and other parts where more waxy, from yellow to red tones.. I scrapped it all together and took some to analysis. My motivation to do this was to concentrate all the non-DMT alkaloids to analyse them. The results: 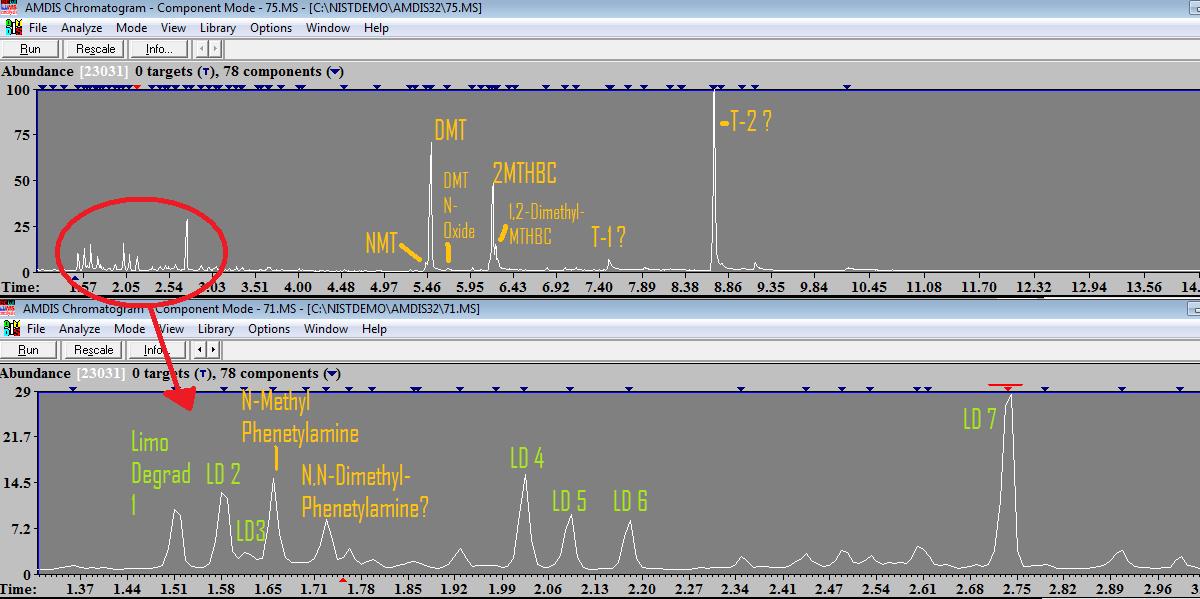 There are several limonene impurity/degradation/Oxidation products, labelled as LD 1-5. They are products such as D-Carvone, trans and cis-carveol, etc.. Then there are 2 substances which came really as a surprise, N-Methyl-Phenetylamine (which matches the same retention time as what was found in Acacia acuminata), and one substance which seems to be N,N-Dimethyl-Phenetylamine (unconfirmed...) Its not contamination because the samples before were not anything related to phenetylamines.. So it really seems mimosa has some phenetylamines in very very small quantities! I would like to repeat this to confirm, maybe Ill get someone else to do this water wash and then analyse it. The other interesting thing is that this water wash concentrates 2-MTHBC which doesnt seem to precipitate much with FASI. So in the end with this wash, instead of 1% or less, its in a ratio of around 3:2 DMT:2MTHBC. Then there is T1 which is clearly a tryptamine-like substance but I cant know exactly, but NIST suggests with 72% chance that its N-Acetyl-Tryptamine ? I will take a look at the mimosa methanol soak to see if I see this small peak there too.. I wonder if its some artifact of analysis/extraction or if its some yet-undetected tryptamine in mimosa... And there is the highest peak (T2) which is some unknown substance, I cannot identify it.... It seems to have a mass weight of 284 (or very close), but I have no idea what it is. Lastly, there is a peak just after DMT with a mass weight of 204 which, due to another test I did that I will post sometime soon, I am pretty confident it is DMT N-Oxide. I have evaporated the water wash with a hot hair drier, so its reasonable that both the limonene traces and some DMT would oxidize.. What is interesting is that this is the first evidence AFAIK that DMT N-Oxide can be detected with gas chromatography... When adding hydrogen peroxide to DMT and testing with GC-MS, this peak appears even stronger (but interestingly enough, there are several more peaks appearing, so it seems peroxide not only oxidizes to DMT N-Oxide but probably also breaks dmt down into other compounds.. but more tests are needed to confirm this) As always, im uploading the .MS file for others to check the work, and later when I have time Ill upload the mass spectra of the doubtful compounds (potential phenetylamines and n-acetyl tryptamine)...
|
|
|
member for the trees
  
Posts: 4003 Joined: 28-Jun-2011 Last visit: 27-May-2024
|
..fascinating work endlessness, thanks..i wonder what the hell 'T2' is? i wanted to be as 'crude' as possible with the Acacia acuminata extract to get an idea what else was in there.. Dimethyl-phenethylamine was apparently detected in Acacia rigidula (though many of the findings in the Clemment paper have been questioned) N-acetyl-tryptamine was reported in one or two Prosopis (mesquite) species.. ..so, no 5-hydroxy-tryptamine showing up? (as in other studies) or maybe this is in leaves.. it would be good to chromatograph and bio-assay 2MeTHBC to look at what it does do, as it seems to be a lot of places that DMT is.. . ..you sure got some 'jungle' spice in the GC-MS this time..! 
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 22-Nov-2025 Location: Jungle
|
nen888 wrote:..fascinating work endlessness, thanks..i wonder what the hell 'T2' is? i wanted to be as 'crude' as possible with the Acacia acuminata extract to get an idea what else was in there.. Dimethyl-phenethylamine was apparently detected in Acacia rigidula (though many of the findings in the Clemment paper have been questioned) N-acetyl-tryptamine was reported in one or two Prosopis (mesquite) species.. ..so, no 5-hydroxy-tryptamine showing up? (as in other studies) or maybe this is in leaves.. it would be good to chromatograph and bio-assay 2MeTHBC to look at what it does do, as it seems to be a lot of places that DMT is.. . ..you sure got some 'jungle' spice in the GC-MS this time..!  Thanks for the feedback nen! Indeed thats what I was thinking, if anybody wants the real "jungle", this method of washing the limo after retrieving DMT from FASI, concentrates much better the non-dmt alkaloids than other methods commonly used like xylene evap or even doing several naphtha washes on the xylene evap jungle. Of course, then we're in untreadded territory here regarding pharmacological activity and toxicity too, specially before we identify what this other main peak is. As for T2, I have no idea what it is, I cant find anything with similar MS, here's the picture of it: 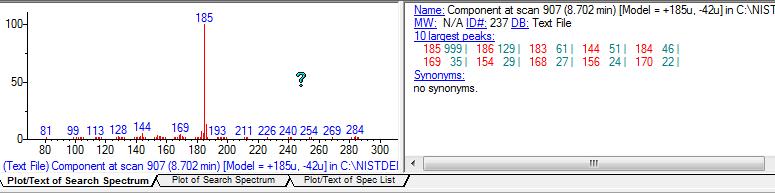 As for the N-Methyl-Phenetylamine, Im pretty sure (as much as I can be with my lack of experience with analytical chem), the spectra is very similar with a standard, here: 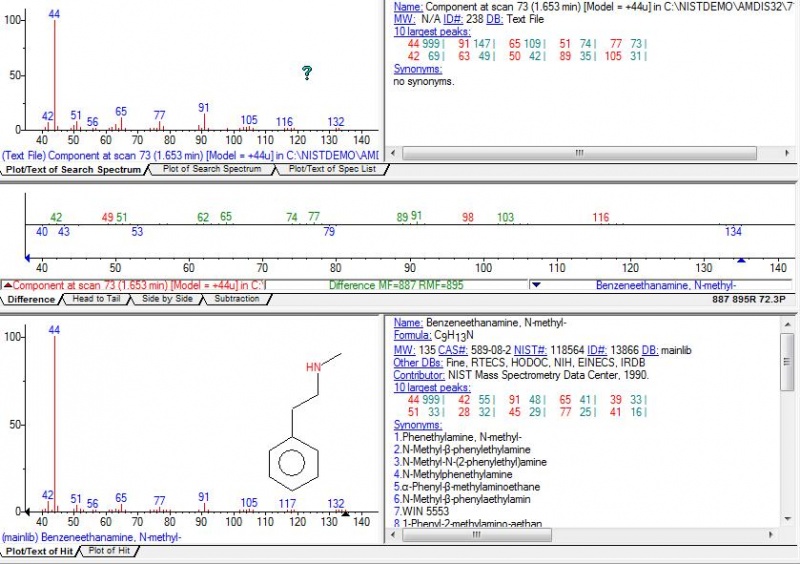 The other unknown phenetylamine, its pretty hard to say.. It has the spectra that isnt really like n,n-dimethyl-b-phenetylamine (I specially notice the lack of fragments under 58 in the literature values of this substance), and NIST gives a 33% possibility.. At the same time it has similarities to methamphetamine because it does have the lower weight fragments (though I doubt this compound would be there? and anyways the match is under 10% chance according to NIST).. Maybe its yet another unknown phenetylamine. Here's the spectra, VS nn-dimethylphen and methamphet... 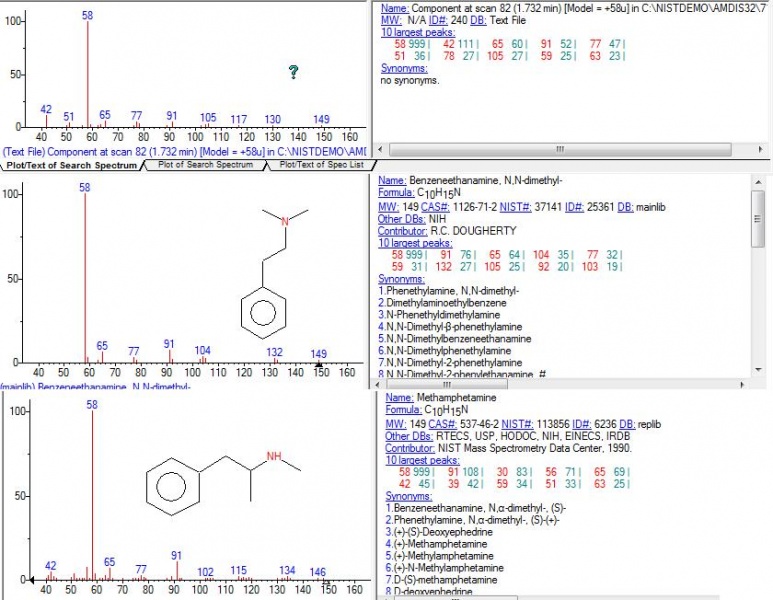 By the way, one comment for dozuki... Doz, check out the TLC plate on the top of this last page. Notice line 7, which corresponds to this wash that we're talking about. Do you notice on top, in that streak of fumaric acid? Well if you look well, appart from the fumaric acid, there is a distinct clear spot there, that shines on UV. Im wondering if thats the same substance youve been getting, with a higher Rf than DMT.. Also remember that Gardner in that publication Shaolin found, speculated that might be 2MTHBC.... This would make sense that it shines under UV (yours did too?), and that its quite more distinct spot now than ever before (and indeed GC-MS shows its much more concentrated). But how this would relate to your finding that it breaks down after a while, I dont know... Maybe we're talking about 2 different substances?
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 22-Nov-2025 Location: Jungle
|
Hah, and some more evidence that these phenetylamine substances are really there in mimosa:
When looking at the mass spectra of mimosa pure methanol soak (post 29, page 2 of this thread), when zooming in to the first minutes of the TIC, I can see two small bumps on the same retention time as the phen substances... They are so so small that AMDIS doesnt even detect both as peaks, it mistakes one of them for 'noise'. It does detect one of the small peaks, at min 1.7505 (versus min 1.73 of this wash for the supposed dimethylPEA), and its such a small peak that the mass spectra of it only detects one main fragment at weight 58. Funny though because this is pretty good confirmation its the same substance, considering that phenetylamine (nn-dimethyl? or whatever else?) is also at pretty close retention time (retention times change slightly depending on the day, temperature, etc) and that it has the base peak at 58. The n-methyl-phenetylamine peak is indistinguishable from noise, to AMDIS program, but I can see its there, a small bump, a couple fractions of a second before.
So its not contamination or similar, there really seem to be phenetylamines in mimosa (!!). I would love to have someone else re-do this wash after FASI so we can test from another source...
|