
DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 22-Nov-2025 Location: Jungle
|
So one happy weekend a couple of weeks ago, Infundibulum, The Traveler and I were hanging out here together and we did a little experiment: Pre-work: Harmalas were extracted from syrian rue, precipitated mostly pure harmine (1 ) by adding sodium bicarbonate until no more color change was seen. This was left to stand for an hour, filtered to retrieve harmine, and then some sodium carbonate was added till no color change was noticed anymore, to precipitate a mixture of harmine/harmaline (2). This mixture (2) was filtered/dried and redissolved in acidic solution. To this solution, sodium bicarb was added to re-precipitate the harmine from mixture, which was filtered, and then sodium carb was added to precipitate reasonably pure harmaline (95%). The reduction: We dissolved the harmaline in some cheap supermarket vinegar, added excess zinc dust (which had been kept in a plastic bag for 2 years, we were affraid it had mostly oxidized and would not work). A lot of zinc dust just stayed on the bottom, and we could notice some very very fine bubbles in the vinegar (which is Hydrogen gas that is formed). We stirred occasionally and let it sit for a couple of hours. Finally, solution was filtered and sodium carbonate added. There was a lot of precipitation. The precipitation was a mixture of alkaloids as well as zinc carbonate. In literature, this reduction is often done with HCl instead of vinegar, and then adding ammonium chloride, and later ammonium hydroxide to precipitate the base alkaloids. Apparently ammonium chloride prevents zinc salts from precipitating, so you only have your THH. But since we didnt really consider consuming and it was just a test, we precipitated with sodium carbonate and had excess zinc carbonate. Zinc carbonate is NOT soluble in alcohols, so theoretically someone could use some ethanol to soak the alkaloid+zinccarbonate mixture and evap that to have alkaloids free of the zinc carb.. To analyze, after filtering this mixture, I soaked it in methanol, which was inserted in The results: 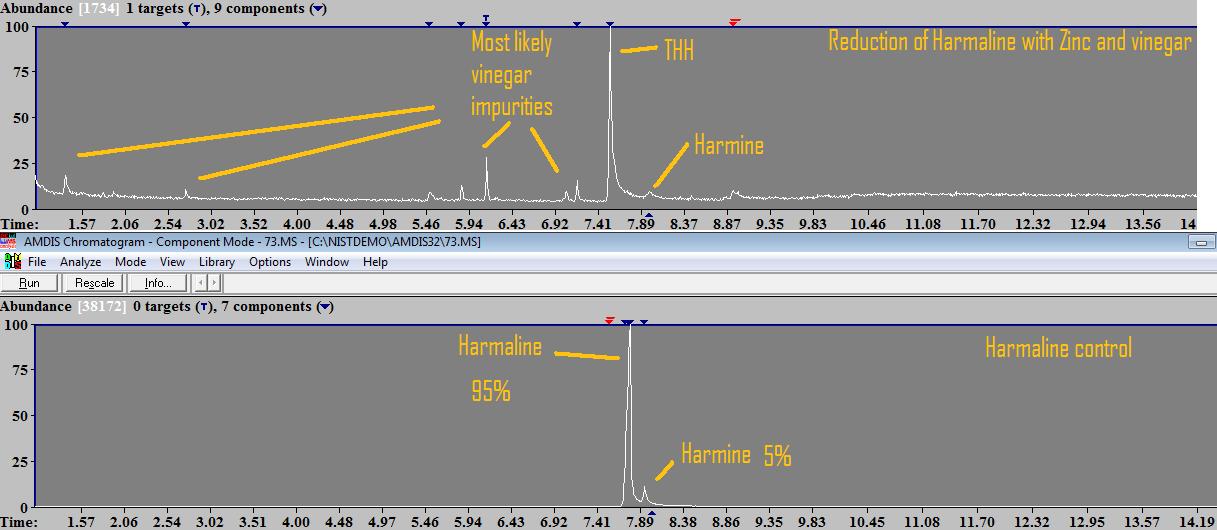 GC-MS results show the conversion from harmaline to THH to be virtually 100%. There are no traces of harmaline detected, only small peak of harmine left (zinc is probably not strong enough to reduce harmine to harmaline but it might be it does, I cannot quantify that peak with the program I have it doesnt "detect" it). There are other small peaks which are very likely the vinegar impurities (acetic compounds... one that seems to be glycerol acetate and other long chain compounds which seem to be carboxylic acids), Im not sure if any of these compounds were created from reduction of vinegar or if they are present in vinegar in the first place, but they are certainly not coming from the harmalas. There is one other small peak which is a phthalate, coming from plastic container used during dissolution of the alkaloids in methanol. Conclusions and questions: People with access to zinc (not zinc oxide or any other form! has to be pure zinc) can reduce their own harmaline into THH easily at home. People need to be careful with hydrogen gas build up (we dont want explosions please!), so let the container vent and work in well ventilated area away from fire sources. There are still a couple of questions to be answered: - Can we find other ways to separate THH from zinc carbonate without the need for solvents? - Does 96% ethanol dissolve zinc carbonate in any significant quantity vs what it dissolves THH? - Can zinc reduce harmine to harmaline too? - What is the toxicity of zinc carbonate? As always, im attaching the raw .MS file from agilent mass spec for those that want to double check my work, as well as the mass spectra of the detected THH peak
|
|
|
|
|

"No, seriously"

Posts: 7324 Joined: 18-Jan-2007 Last visit: 10-Jan-2026 Location: Orion Spur
|
AWesome work endlessness. I'm glad that the nicely looking chemical reaction was actually doing what we expected it to be and hoped for. Kind regards, The Traveler
|
|
|

DMT-Nexus member
Posts: 192 Joined: 09-Sep-2009 Last visit: 18-Jun-2014
|
Excellent! This will definitely encourage some experimentation. Zinc powder isn't hard to find at all, just make sure you get the right grade.
|
|
|
DMT-Nexus member

Posts: 351 Joined: 25-Jul-2009 Last visit: 25-May-2016 Location: Europe
|
On the third question, one could answer that by subjecting the "almost pure harmine" from the first precipitation to the same method, and run both the reaction product and the starting material through analysis.
I also wonder could other easily accessible metals be used? I have been looking at redox tables and while zinc has the value of -0.76 V, Aluminum has -1.66 and magnesium has -2.36 (lower = greater reducing power). Both of those metals can be found easily, aluminium is propably in everyone's home and magnesium can also be sourced from firestarter kits containing it. Am i missing something that would make aluminium or magnesium not suitable for such reductions?
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 22-Nov-2025 Location: Jungle
|
How would you proceed with aluminium? would you break up alu foil in small pieces and just do the same thing, mixing it with acetic acid solution? I can test it out too... Is there anything I should worry about?
I saw aluminium carbonate decomposes into CO2 and aluminium hydroxide.. Maybe aluminium hydroxide is soluble in water so simply washing the (hopefully) THH with water could clean it up nicely... What do you guys think?
Dagger, I got my zinc from a chem supply shop here in town, cant help you much sorry...
And yeah crystalito def the way to answer the harmine reduction question is to do it with harmine, Ill do that when I have some more harmalas to play with, because im basically out now and waiting for rue to arrive.
|
|
|
DMT-Nexus member

Posts: 351 Joined: 25-Jul-2009 Last visit: 25-May-2016 Location: Europe
|
Thanks for the consideration Endlessness. One idea would be to order aluminium powder (as i suspect the experimenters ordered zinc powder) but that would kind of "defy" the purpose of it being almost "step in your kitchen and do it". What you suggested with the aluminium foil could work if cut in small pieces, with the greatest possible surface - in this line of thought it could help using it as small pieces of flat foil (1/4 "blotter" size? Smaller if one can?) instead of rolling it into small balls. Also, aluminium can ozidize easily loosing its "gloss" so use soon after you cut it - a complemetation here would be scrubbing the aluminium to reveal "fresh" metal before cutting and using but that would be complicating the procedure if one can do without it. Personally i have tried putting pieces of regular aluminium foil in HCl and hydrogen gas evolved (checked by "burning"  , which is indicative that the reaction proceeds without aluminium being totally "protected" by the thin layer of oxide that it might have. Of course anyone wanting to try such experiments keep in mind that your aluminium foil must be straight aluminium (i've seen some "extra strength" ones or "non sticky" that have some king of plastic embeded on them). So yes, to keep it simple small pieces of aluminium in the reaction beaker! No other things to worry about, just keep in mind that hydrogen gas will evolve as with zinc so safety procedures for explosive gasses apply. Of course, after the reaction has taken place you can remove any unreacted aluminium foil. I would be really glad if you can test it, if it works with harmaline maybe one could try with harmine also and compare the results to zinc. Also another question , sorry if you mentioned it and i missed it: was the reaction heated? This could speed up the reaction. Some things that would help in the endeavors as far as solubility goes : Solubility calculator of some metal salts : http://chemmac1.usc.edu/bruno/java/solub.html (it gives aluminium carbonate and hydroxide as... insoluble in water) Solubility table of metals http://mrnorton.com/Chem...s/Solubility%20Rules.pdf (has chlorides, acetates, carbonates of metals which can be of use!) And of course the big solubility chart of wikipedia: http://en.wikipedia.org/wiki/Solubility_tableIf other salts are to be examined or solubility in other solvents propably the respective MSDS of each compound could answer the question.
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 22-Nov-2025 Location: Jungle
|
Thanks for the feedback ! I will keep it in mind when/if I can test it again but with aluminium
And no, reaction was not heated... It was really "crudely" done, without any special care to make it some thorough scientific-work... So I was amazed to see the results!
|
|
|
DMT-Nexus member

Posts: 473 Joined: 07-Aug-2011 Last visit: 10-Jan-2014
|
Ah yes very nice work yet again endlessness.
To remove zinc carbonate without the use of solvents hmm... If a Manske works on THH I believe it would be a viable option. I haven't checked the data but I don't believe NaCl will undergo metathesis with ZnCO3 in aq. solutions. Even if it did the solubility of ZnCl2 is immense(4320 g/L (25 °C) from wikipedia).
Or one could add an excess of NaOH to an aqueas solution of THH + ZnCO3 to form freebase insoluble THH and Zn(OH)2. The Zn(OH)2 should be soluble and create a colorless solution in the hydroxide laden solution while the THH should precipitate out. So long as no chelation/coordination complex forms or something I am unaware of.
|
|
|

The Root
 
Posts: 2458 Joined: 02-Jul-2008 Last visit: 27-Sep-2023 Location: The asteroid belt
|
i have been doing the zink hcl thing i posted a while back for some time now - always with success, i didnt post more about it because everytime i did, people said otherwize. some of the nicest thh xtals i have seen grew when a zink/hcl reduction was left over night - as the solution slowly became more basic, they precipped sometimes a ballon is fitted over the top of the vessel to prevent the gas from escaping - but most often its done outside aluminium scares me, powdered elemental zink on the other hand seems friendly enough can someone describe the ammonium chloride proceedure a bit better - it worked fine but i felt i like i was operating with less facts about whats going on than required. professor8 posted a tek a while back that uses ascorbic acid, id be interested to see the outcome analysed antrocles wrote:...purity of intent....purity of execution....purity of experience...
...unlike the "blind leading the blind". we are more akin to a group of blind-from-birth people who have all simultaneously been given the gift of sight but have no words or mental processing capabilites to work with this new "gift".
IT IS ONLY TO THE EXTENT THAT WE ARE WILLING TO EXPOSE OURSELVES OVER AND OVER AGAIN TO ANNIHILATION THAT WE DISCOVER THAT PART OF OURSELVES THAT IS INDESTRUCTIBLE.
Quote: ‹Jorkest› the wall is impenetrable as far as i can tell Quote: ‹xtechre› cheese is great He who packs ur capsules - controls your destiny.
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 22-Nov-2025 Location: Jungle
|
Phlux- wrote:i have been doing the zink hcl thing i posted a while back for some time now - always with success, i didnt post more about it because everytime i did, people said otherwize.
some of the nicest thh xtals i have seen grew when a zink/hcl reduction was left over night - as the solution slowly became more basic, they precipped
sometimes a ballon is fitted over the top of the vessel to prevent the gas from escaping - but most often its done outside
aluminium scares me, powdered elemental zink on the other hand seems friendly enough
can someone describe the ammonium chloride proceedure a bit better - it worked fine but i felt i like i was operating with less facts about whats going on than required.
professor8 posted a tek a while back that uses ascorbic acid, id be interested to see the outcome analysed
Phlux can you explain this about the thh crystals? What do you mean you left the reduction overnight? without filtering the excess zinc? and the THH precipitated without adding a base? or.. What base did you use, and how did you separate it from the excess zinc salts that also precipitate? Ill try to find the original source for the ammonium chloride claim and post, but IIRC, they were not specific about th amounts or anything, just said "adding ammonium chloride increased yields etc something" By the way phlux, what about bioassaying that thh, did you? And what do you think of the post of this person who said thh does NOT orally activate dmt: https://www.dmt-nexus.me...aspx?g=posts&t=27572 ?
|
|
|

Kalt und Heiß, Schwarz und Rot, Kürper und Geist, Liebe und Chaos
 
Posts: 4661 Joined: 02-Jun-2008 Last visit: 30-Apr-2022
|
Nice one endlessness! It's really nice to see that the reduction works with pretty much close to 100% efficiency with just store-bought vinegar and within 2 hours (as opposed to using copious amounts of HCl as suggested in teh literature... I am surprised that the reduction was performed to completion, as opposed to the 50-70% yields reported in the literature. Maybe there's something about using vinegar as the acid (and not HCl) that contributed to that? Either way, there seems to be more advantages to using vinegar when at least it comes to harmaline reduction. The next step post-reduction is to make the solution basic and precipitate the freebase THH; but the problem is that whatever base one uses, zincs will precipitate; if one uses ammonia, NaOH, KOH then zinc hydroxide will precipitate, as it is insoluble in water. If one uses sodium carbonate then zinc carbonate (and to a much lesser extent zinc hydroxide) will precipitate. Zinc hydroxide and zinc carbonate should not be soluble in ethanol (I nave hard time believing that a water-insoluble inorganic salt would dissolve in ethanol!), so ethanol pulls should get just the alkaloids out. Too bad that harmala freebases are not much soluble in ethanol either... Another approach worth trying (and this is where I'd put my money) would be to first use any available base to precipitate the zinc salts and THH freebase, then collect precipitates and retrieve THH with vinegar or better carbonated water; All the zincs will remain insoluble and be left behind while THH will be collected as its acetate or carbonate salt, ready to be re-precipitated with base. As for harmine, we have no evidence for it being reduced to harmaline (and then to THH). We just assume from the FV THH fiasco that it is not reduced by zinc. Either way, it'll be great to put this into taste, so we have hard data on this issue! Overall, I feel that we're coming a step closer to confidently making THH safely at home and soon people will be able to do their own experience experiments (e.g. like does it orally activate dmt?) Phlux- wrote:i have been doing the zink hcl thing i posted a while back for some time now - always with success, i didnt post more about it because everytime i did, people said otherwize.
some of the nicest thh xtals i have seen grew when a zink/hcl reduction was left over night - as the solution slowly became more basic, they precipped
sometimes a ballon is fitted over the top of the vessel to prevent the gas from escaping - but most often its done outside It's true that over time zinc will react with water to form zinc hydroxide which, will raise the pH and precipitate THH. But I wonder, how did you further clean up your product? The nice crystals you're seeing might as well be zinc salts or similar... Also, to what extent are you confident that your reduction is complete? I remember you also did that zinc reductions way back but you dod not provide any hard evidence other than colour changes and subjective effects upon administration. I also remember you claimed (please correct me if I'm wrong) that harmine is also redudible to THH, again based on colour and fx changes) but this is still a disputed and unresolved issue. Need to calculate between salts and freebases? Click here! Need to calculate freebase or salt percentage at a given pH? Click here!
|
|
|

DMT-Nexus member

Posts: 14191 Joined: 19-Feb-2008 Last visit: 22-Nov-2025 Location: Jungle
|
Yeah I was also so surprised at how perfect the conversion was! Whats your take on using aluminium foil, inf? Do you think that could work, or do you see any potential downfall on this alternative?
Also, Im interested in what you said about redissolving THH.. So youre saying that if I was to use some warm vinegar, it would NOT dissolve the zinc carbonate? Wont the carbonate in zinc react with acetic acid and form zinc acetate which will be more soluble in solution?
|
|
|

Kalt und Heiß, Schwarz und Rot, Kürper und Geist, Liebe und Chaos
 
Posts: 4661 Joined: 02-Jun-2008 Last visit: 30-Apr-2022
|
endlessness wrote:Whats your take on using aluminium foil, inf? Do you think that could work, or do you see any potential downfall on this alternative? It could work - the main test to be done is to chop up aluminium and soak it in moderate to strong acid, such as vinegar and HCl. If it bubbles, then it's worth trying it out - the bubbles are hydrogen, the mediator of the reduction reaction. The only concerns are then aluminium contamination of the final product, which may or may not be that healthy. Definitely worth researching further. endlessness wrote:Also, Im interested in what you said about redissolving THH.. So youre saying that if I was to use some warm vinegar, it would NOT dissolve the zinc carbonate? Wont the carbonate in zinc react with acetic acid and form zinc acetate which will be more soluble in solution? It is tough to answer because zinc carbonate will react with acetic acid to give the very soluble zinc acetate, and then zinc acetate will react with sodium carbonate to give the very insoluble zinc carbonate.... It appears to be difficult to find a fair balance if one really needs to play with acetic acid and sodium carbonate for the conversion and isolation, that is why it is worth trying with carbonated water, which is unreactive to zinc carbonate. I'll try and put some more thought in case there's another way out... Need to calculate between salts and freebases? Click here! Need to calculate freebase or salt percentage at a given pH? Click here!
|
|
|

Faustian Phytochem Investigator
 
Posts: 194 Joined: 31-Oct-2011 Last visit: 29-Nov-2025 Location: Oaxaca
|
This is all really nice work!!! Makes me want to play more with the beta-carbolines, but I have a list of stuff to look into first. Does anyone have the original refs. that Trout quoted about this? I believe they are Naranjo 1967, and Siddiqui et al. 1983?
|
|
|

Stiletto Stoner

Posts: 1132 Joined: 18-Nov-2008 Last visit: 15-Mar-2015 Location: Blazin'
|
Dozuki wrote:This is all really nice work!!! Makes me want to play more with the beta-carbolines, but I have a list of stuff to look into first. Does anyone have the original refs. that Trout quoted about this? I believe they are Naranjo 1967, and Siddiqui et al. 1983? Ethnopharmacologic Search for Psychoactive Drugs 1967 @Erowid (80 MB) "Ayahuasca, caapi, yage. Psychotropic properties of the harmala alkaloids" by Narajo is at page 413. "Studies in harmine series of alkaloids" is attached. "Part 1. Derivatives of tetrahydroharmine" starts at page 10. Got GVG ? Mhm. Got DMT ? Pandora wrote:Nexus enjoys cutting edge and ongoing superior programming skills of the owner of this site (The Traveler), including recent switching to the .me domain name. I'm still, I'm still Jenny from the block Simon Jester wrote:"WTF n00b, buy the $100 vapor pipe or GTFO" Ignorance of the law does not protect you from prosecution
|
|
|
DMT-Nexus member

Posts: 351 Joined: 25-Jul-2009 Last visit: 25-May-2016 Location: Europe
|
Possible contaminations by metal compounds can be worrying, but of course it depends on the metal, the quantity and the frequency of use of the extract. Phlux why are you afraid of aluminium? Zinc can have toxicity too and there are cases of poisoning, aluminium can be toxic as well but the literature as far as i know describes it as relatively not toxic (think of maalox and other antacids containing it, not something you would like to drink everyday all day long but still its not that toxic afaik).
Still, its best if we can find a way to clean the product of metal compounds. Alcohols were mentioned but i wonder if THH is soluble in it and if we might stumble upon "harmala red" situations.
Also as far as metals go, keep in mind that Magnesium could be tried as well -although aluminium is easier to find. If magnesium compounds have different solubility profile than the ones of the two other metals it could be given a thought (if the metal contamination is easier to avoid).
|
|
|

Faustian Phytochem Investigator
 
Posts: 194 Joined: 31-Oct-2011 Last visit: 29-Nov-2025 Location: Oaxaca
|
Thanks again Shaolin, you rock!!
|
|
|
DMT-Nexus member

Posts: 473 Joined: 07-Aug-2011 Last visit: 10-Jan-2014
|
Infundibulum wrote:...
The next step post-reduction is to make the solution basic and precipitate the freebase THH; but the problem is that whatever base one uses, zincs will precipitate; if one uses ammonia, NaOH, KOH then zinc hydroxide will precipitate, as it is insoluble in water.
Quote:If excess sodium hydroxide is added, the precipitate of zinc hydroxide will dissolve, forming a colorless solution of zincate ion: Zn(OH)2 + 2OH- → Zn(OH)42-. This property can be used as a test for zinc ions in solution, but it is not exclusive, since aluminum and lead compounds behave in a very similar manner. Unlike the hydroxides of aluminum and lead, zinc hydroxide also dissolves in aqueous ammonia to form a colourless, water-soluble ammine complex. taken from - http://en.wikipedia.org/wiki/Zinc_hydroxideThat's why I said what I did in my last post. So instead of sodium carbonate, NaOH could be used would make an uneccessary mess ad battle equilibrium. Haven't checked the theoretical microspecies for THH but I doubt high pH will present much of a problem. Also note the water soluble amine complex. edit - here I'll lay down a possible procedure... Precipitate with Na2CO3, collect zinc hydroxide laden THH, wash twice with a fairly saturated NaOH or KOH solution. Ideally these solutions can be recycled to repeat the process.
|
|
|

Kalt und Heiß, Schwarz und Rot, Kürper und Geist, Liebe und Chaos
 
Posts: 4661 Joined: 02-Jun-2008 Last visit: 30-Apr-2022
|
^^ Good to know! but why do you... InMotion wrote:edit - here I'll lay down a possible procedure...
Precipitate with Na2CO3, collect zinc hydroxide laden THH, wash twice with a fairly saturated NaOH or KOH solution. Ideally these solutions can be recycled to repeat the process. ...instead of just precipitate the fb THH with NaOH from the very beginning?  What we're trying to do is to just use sodium carbonate instead of NaOH, since the former is far more easily obtainable. In theory, if one has NaOH he reduces in vinegar then bases with NaOH. I guess the only problem would be that if one needs a high NaOH concentration to keep the formed zinc hydroxide in solution he may be harming the THH. We really (and I mean, really! do not know whether beta carbolines and especially THH are stable at this high pH values! So back to the drawing board, unless one is willing to test how stable THH is at very high pH values.... Need to calculate between salts and freebases? Click here! Need to calculate freebase or salt percentage at a given pH? Click here!
|
|
|
DMT-Nexus member

Posts: 473 Joined: 07-Aug-2011 Last visit: 10-Jan-2014
|
last edit - Personally I wouldn't use carbonate as a source of base. I'd stick to the procedure and use ammonium chloride and ammonia to form I presume the zinc amine complex(which I thought the haulting of precipitation was governed by equilibria before). Or maybe toy with only using NH4Cl(or similar ammonium salt) and a base such as NaOH running the reaction 'dry' to see if the precipitate went into solution or not. I try not to work with weak acids in conjunction with weak bases though I understand the desire to do so, due to ready availability. In my second post I was assuming that Na2CO3 would form less of the Zn(OH)2, which was based on absolutely nothing. Hydroxide is likely a good friend in this instance. It is interesting that the results were so clean using house-hold vinegar as the acid. If only I could remember my qualitative analysis lab's  . I'll look into the high pH value thing, I don't see an obvious reason for instability off of the top of my head. It would also be good to know if THH will precipitate in a saturated NaCl solution(ala 'manske'  .
|