The sad story of Bufotenin:10 years ago 69ron wrote Bufotenin is the most colourful and least psychedelic cousin from the Trio of DMT, 5-MeO-DMT and 5-OH-DMT (Bufotenin). It would give strong CEV and OEV with patterns and colours and would not be harsh to smoke when pure. As it happened quite some times before that he made exaggerating reviews on a substance, this may have needed more verification by others. Many people tried, but reported to only extract amber to black stuff from A. colubrina seeds, which give a harsh vapor with strong vasoconstriction - also reported by 1950's early Bufotenin trials who stated them inactive. It had to come until Jonathan Ott reported some visionary effects of Bufotenin decades later, but still it was believed that you would mostly get unpleasant effects, as you'd more likely smoke an Alkaloid mix with a lot of nauseating substances.
So until now 69ron's praise of Bufotenin was not likely to be proven, but still his idea of extracting Bufotenin in its liquid form with Xylene seems like the final key to get a pure crystalline compound. So when I finally had some truly pure material I was excited to finally try it, but mostly ended up with nauseating vapor and vasoconstriction in the first trials, but still at any time being super harsh and non-smokable even with sper gentle vaporization. Back in the days he stated to use a test tube with a straw and heated from below. As the material collected at the bottom when heaten up, I guess this method would also produce a lot of charred products, making it far from enjoyable to smoke.
Therefore I believe probably many people who have smoked Bufotenin and gave it a bad review may have still smoked high purity Bufotenin and believed their dark Bufo may just have been full of 'plant poisons' due to the colour - somehow the same story like the belief a dark DMT fraction might hide a multitude of mysterious full-spectrum alkaloids.
Evaporation of Bufotenin FreebaseI measured the Vaporization Profile of Bufotenin some time ago and here we can directly see that vaporization of Bufotenin is really unconvient. Instead of Vaporizing to more than 90 % like DMT and 5-MeO-DMT something happens, which instead burns like 50 % of the Bufotenin. That reaction must be linked to the OH-group as seen
here and is most likely the same reaction that Psilocin in Mushrooms can undergo, which causes their blue colouring and instability when not properly stored. The reaction is an oxygen mediated coupling of single Tryptamines to Oligo- to Polymers. In the case of Psilocin a bigger molecule conjugated with pi electrons (basically double bonds) extends its absorption from UV-only (being formerly colorless to our eyes) into the visible range, though becoming coloured = blue. The coupling positions of Bufotenin enable only a conjugation which is sterically more hindered than for Psilocin, that might be a reason why at room temperature Bufotenin stays stable forever. Furthermore Psilocin is also in touch with corresponding enzymes in the Fungi, that will promote this reaction even more at Room Temperature. As a consequence this reaction only takes place at high temperatures, like when vaporizing. It seems the threshold is reached at 170 °C, any Bufotenin will quickly become black above this point. The polymerization product will not vaporize anymore, hence the stop of any evaporation. Still traces of it might also vaporize upon heating, then if you inhale them they irritate your lungs like hell. That's why I can only hold 2 mg of properly vaporized Bufotenin for like 3 seconds, making it impossible to ever get any true Bufo experience. Now here you will see a picture of the vaporization of Bufotenin if you would just directly smoke it and the reaction that possibly makes evaporation so inefficient plus irritating. Keep in mind that is only a theory, but at least a quite reasonable one:
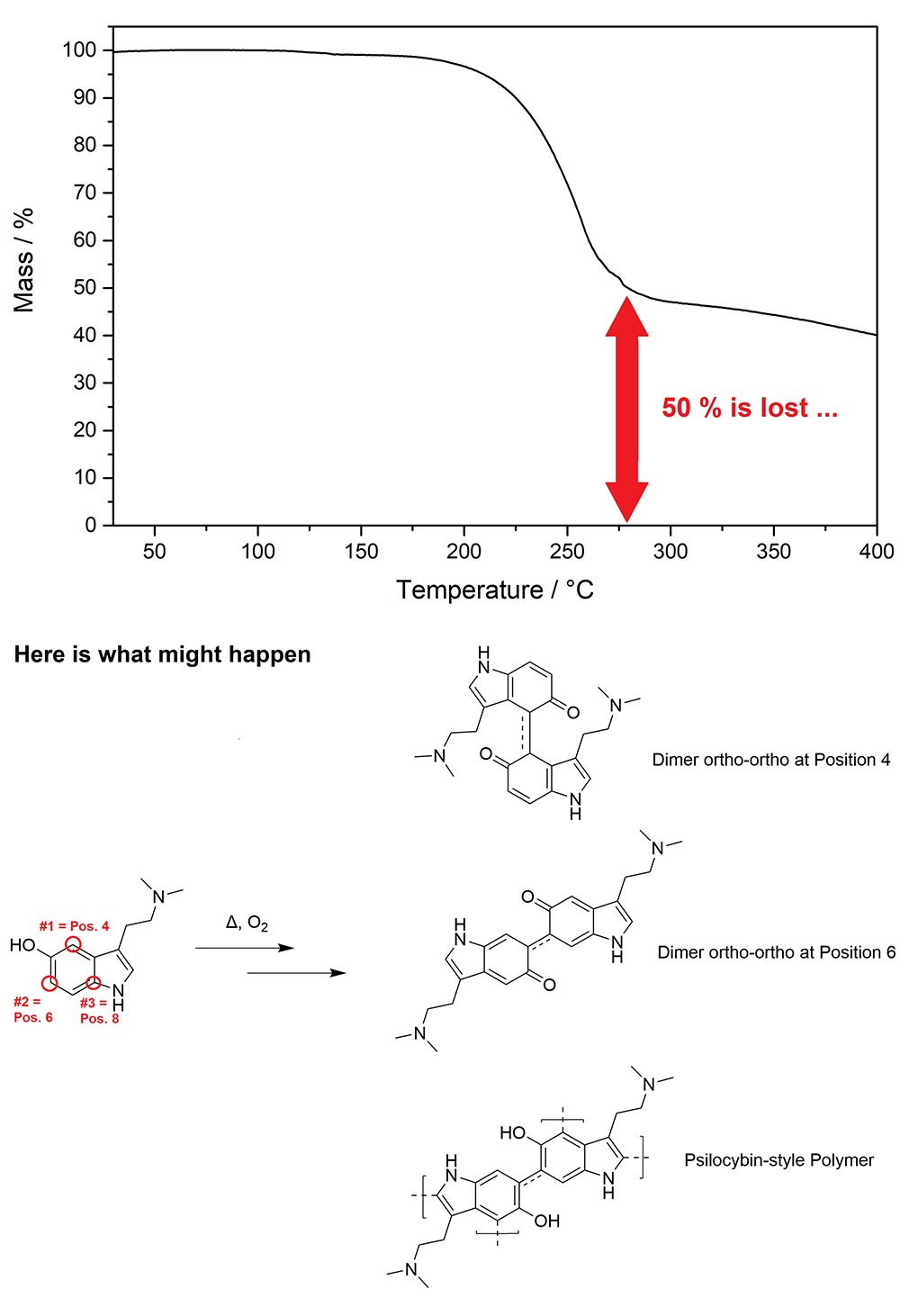
Bufotenin might be coupled
like Psilocin, but 1 out of 3 positions are blocked. In case of a reaction a chinon-like polymer is created, which is coupled through a positioning which is labelled as 'ortho' in aromat nomenclature. Obviously there also could be mixes between the both Dimers shown above like 4+6 and 6+4. The position #3 would be 'para' and an enzyme-free and therefore thermodynamically-controlled coupling at this position would be the most likely. As that is not possible and the other 2 sites probably have a higher energy barrier due to steric hinderance from the Oxygen the corresponding reaction needs 170+ °C to occur, so only happens when vaping it. Maybe at first only Dimers are formed and then after 280 °C all that stuff is helplessly charred down to Polymers. But that would be just some speculation.
Now I guess in any way this cannot be prevented, hence I have no idea how to properly smoke Bufotenin ... Furthermore if eaten 100 mg only produce a weak effect (like 1 g Mushrooms) and also induce nausea. Seems pretty much like a dead end with Bufotenin becoming the unbeloved sibling out of the Trio. Bufotenin may be just a bitch in vaporization and also annoying to crystallize ... so what can be done now?
Solution 0 (not really a solution ...):
Evaporating Bufotenin in an inert atmosphere without Oxygen
First I wanted to make an easy test how to get something like a proof that the problem is an oxygen-induced assembly of non-vaporizing, probably higher molecular weight substances, which is causing the whole trouble. Here is a super easy experiment I was doing: Just conduct the vaporization in Nitrogen. The oligomerization requires the Phenol to Oxidize to the Chinon-like structure. Oxygen is the go-to molecule to promote Oxidations (I guess that's where the name is from). By conducting the TGA experiment in an inert atmosphere, there is no possibility to promote the reaction at least with Oxygen. So here is the result:
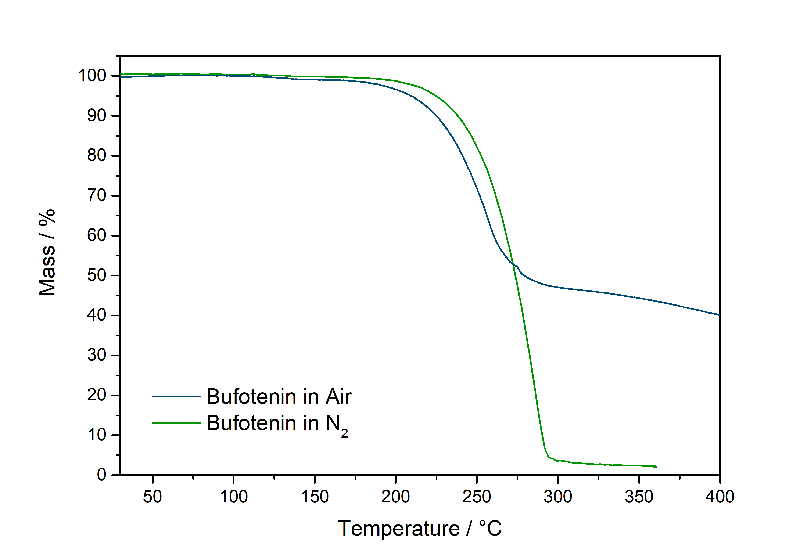
Hooray!! FULL evaporation - only 2 % Bufotenin remaining. So we can directly see that removing the Oxygen all molecules will make their way into air. Now that is all cool and stuff, but I guess not many people here will fly to space to vaporize their Bufotenin in zero-G and vacuum (although temperatures for vaporization will be conveniently low). Therefore this is not more than a deeper look into the process in general. So what can we do to still experience this substance, if we know that the problem is an oxygen-promoted reaction, that blocks Bufotenin from vaporizing, while irritating the lungs with charred reaction products?
Solution 1:
Conversion of Bufotenin into the corresponding salt
Many evidences recently came up that Tryptamine salts of a certain kind can be vaporized at actually just a few °C higher than the Freebase, being just a 10 % more temperature for DMT for example. A general discussion about this and which salts are working and why can be found
here. The interesting thing here is even more that these dont seem to be too much more harsh than the freebase. Now you may ask:
Ok ... cool so is this just some kind of scientific oddity or why else should I care? Indeed in the first place it is quite astonishing, but I admit it would normally not make too much of a difference to the Nexus' users. But now as there was some discussion about this general Bufotenin problem, consider this:
Considering that ALL salts of ALL tryptamines vaporize in the same pattern, which would then also be valid for Bufotenine
--> How about utilizing the salt formation to introduce a charge to the Bufotenin molecule, which will
maybe stop polymerization by pushing away any molecules of similar charge? The impact of this on the initial problem is written further down. (Make yourself ready for some epic powerpoint showcase)
To see if that will make any difference I created some Bufotenin Benzoate and repeated the same Test ... here is the result:
So what you can see here: Quite a good vaporization of up to 70 %. Not bad actually, considering that for DMT and 5-MeO-DMT we are also just above 90 %. Maybe there is still a small chance for creating Oligomers. But highly-charged polymer-chains are a somewhat thermodynamically disfavored thing. In industry there is many stuff like sulfonated polystyrene, but in those cases probably not EVERY side chain carries a charge - like it would do here. High charge density on a small spot is disfavored in nature, therefore even if the reaction may still occur we might have only oligomers instead of polymers. Even better:
Probably the charged side-products will not vaporize at all, so traces of them can't irritate your lungs. Will have to verify soon with a Bioassay (funny word). So just from the experimental results, I'm quite convinced that this method will actually render Bufotenin smokable to a drug than can be easily enjoyed finally.
See experimental results here.
Solution 2:
Chemical conversion of Bufotenin to block Chinon-formation
So the key to all this evil is that Phenolic compounds can be oxidized to a keton state. That is also used in many anti-oxidants (they oxidize themself instead of the substances they protect), which are also often derived from phenols. Therefore any modification that will stop the 5-Oxygen from forming a double bond will effectively block this pathway. This can be demonstrated with 5-MeO-DMT: A demethylation would be needed and this requires fancy chemicals (H2/PtC or BBr3) instead of just heat. You may ask how is a true chemical conversion then still a solution to experience Bufotenin. The answer is you might use a substitutent for blocking, which is easily chipped off in the body like Acetic Acid = Ester formation. Therefore creating 5-Acetyl-DMT from 5-OH-DMT (Bufotenin) might be a way to block chinon-formation, while the (proposed) Pro-Drug will be transformed back to Bufotenin upon ingestion. See Scheme below (just ignore the quaternary Amine at MeO

):
Solution 3:
Create a Bufo-infused E-Juice for Vaporizers
Loveall has
experimented a lot with E-Juice and also with some possible Tryptamin salts, which may have a better shelf life when stored in these mixtures than Freebase. A benefit of this might be to aerosolizing the Tryptamine in the E-Juice instead of vaporizing it directly. Therefore this method might even be performed with the picky Freebase. I dont have a suitable device for proofing, but if anybody else has they might report here:
Loveall wrote:How about dissolving bufotenine or bufotenine salt in PG and vaping it (aerosolizing)?
Loveall wrote:The e-juice becomes small dropplets can carries the drug still dissolved in it.
Summary:Even though being only a theory, experiments seem to show that Bufotenin might undergo the same oligomerization like Psilocin, but only oxygen mediated at > 170 °C. As it might seem impossible to conduct any vaporization for personal enjoyment without Oxygen, it looks like vaporization of the salt is shutting down this process by a decent amount. Also a chemical conversion blocking that pathway in general might also help to stop this reaction to occur, hence finally this compound could be vaporized just like other Tryptamines.