
dysfunctional word machine

Posts: 1831 Joined: 15-Mar-2014 Last visit: 11-Jun-2018 Location: at the center of my universe
|
I don't see the point of the patent. Why make fumarate salts? HCl salts of harmalas are non-hygroscopic and highly stable. Also, for medical use, HCl salts in general are perfectly acceptable AFAIK.
If you worry about NaCl contamination after salting, just dissolve in dry ethanol. The NaCl will not dissolve, the harmala HCl salts are quite soluble in ethanol (though not as soluble as in water.) Harmala HCl crystals can be recovered through evaporation of the ethanol.
|
|
|
|
|

DMT-Nexus member
Posts: 212 Joined: 16-Oct-2016 Last visit: 15-Jun-2023
|
Hey guys, after getting a litle off topic, let's get back to the verification of the article's protocols: This is what I started right now: a hydrogenating environment of 3018mg of (partly recycled) Zn-powder in 100ml of acetic acid 7%. To this mixture was added 3000mg of DHH as obtained from the pH-metrical separation following VDS. I plan on letting this system react at room temperature for about 24h. In search of the holy grail...  I'll keep you posted! An1cca attached the following image(s):  3g Zn - 100ml vinegar 7% - 3,000g DHH.jpg (84kb) downloaded 394 time(s). Color at start of reaction.jpg (40kb) downloaded 394 time(s).
|
|
|

DMT-Nexus member
Posts: 4031 Joined: 28-Jun-2012 Last visit: 05-Mar-2024
|
An1cca wrote:Wow Jees, hell breaks loose in that little recipient! An ideal setting for some heavenly THH  The idea was to get the reaction time down. This is still an open question when it is done enough. The microscope might shed light on that matter. Have you noticed that the THH crystals on page 24 look very similar to those of harmine? Only when they are not recrystallized or not sublimated they form the better distinguishable spherical agglomerates of page 23. I'm getting interested in a microscope also  Any good ideas to watch out for? TLC can help too. An1cca wrote:...Jees, if you have some combined harmine+THH left, you could try a reprecipitation from vinegar with sodium bicarbonate to separate both alkaloids. This would validate protocol 4. If you don't have enough left, I think I will have some in a few days, no worries... I've only 470 mg left of the mix, too less for tests but good for 2 pharma sessions. I'm in the middle of other stuff too now, but I enjoy very much your exiting progress  Soon I'll be reducing some very old bark deemsters n-oxide, and will use ammonia to base. Previous time a load of ZnOH interfered with the process. Will report when done. Thanks pitubo for the chemical adds  ijahdan I had read you wrong there for a sec, I stand corrected 
|
|
|

DMT-Nexus member
Posts: 4031 Joined: 28-Jun-2012 Last visit: 05-Mar-2024
|
That 100 ml standard vinegar 7% and Zn that was gassing in the little movie, then choosing sodium bicarbonate to base with  , it took an insane amount to get to pH 8.53 Maybe that brand's sodbicarb is full of a filler? Jees attached the following image(s):  badumtsss.jpg (123kb) downloaded 375 time(s).
|
|
|

DMT-Nexus member
Posts: 1893 Joined: 18-Jan-2008 Last visit: 26-Sep-2023
|
Wow quick fire thread enjoying watching the progress.
Re:sodium bicarb , it has a hydrogen element that aids in
keeping the pH down as opposed to sodium Carbonate. Iirc sodium bicarbonate acts a buffer in many systems
organic and otherwise.
|
|
|

DMT-Nexus member
Posts: 4031 Joined: 28-Jun-2012 Last visit: 05-Mar-2024
|
I've never worked with sodbicarb for basing before, I just pored some powder in and stirred long enough. It was like it wouldn't dissolve and pH wouldn't rise quick either. This is not they way to work with it I suppose. So I guess one has to make a hot saturated solution and drop that in?
|
|
|

DMT-Nexus member
Posts: 212 Joined: 16-Oct-2016 Last visit: 15-Jun-2023
|
DreaMTripper, Jees, I also think some buffering is going on. I stopped adding sodium bicarbonate at pH 7,5 because I noticed it became difficult to get it all dissolved and I wanted to weigh the amount of zinc carbonate that had precipitated without any surplus of bicarbonate. As it turned out, above that pH, no more zinc carbonate was precipitated, indicating that there is no need to overkill the solution with bicarbonate. Lacking a pH-meter and when there's no more need for quantification, a little bit of undissolvable bicarbonate at the bottom might be the thing to aim for. That way, not much valuable mother liquor will stay behind around solid bicarbonate. Jees, I needed quite a lot of bicarbonate as well, will measure it when DHH reduction is finished.
I was lucky to get my hands on a (children's) microscope. Can 't wait to put some extracts on a slide....
|
|
|

DMT-Nexus member
Posts: 4031 Joined: 28-Jun-2012 Last visit: 05-Mar-2024
|
I took the little liquid left & trough a filter, based with NaOH and all kept clear, as expected because of the above post.
When they say "base with sodbicarb" then things look straightforward and you think 8.5 is in reach but its actually not so simple.
One would think: if there is some undissolved at the bottom, it's no use to add more and I have already reached max potential pH possible for sodbicarb, but this is not the case.
Hypothesis at hand: the moment you are sure that some undissolved layer of sodbicarb forms at the bottom, then you can also be sure that all ZnCO3 has precipitated. An1cca's test was indicating OK, but I would like to redo this test to re check.
|
|
|

DMT-Nexus member
Posts: 212 Joined: 16-Oct-2016 Last visit: 15-Jun-2023
|
Jees wrote:Hypothesis at hand: the moment you are sure that some undissolved layer of sodbicarb forms at the bottom, then you can also be sure that all ZnCO3 has precipitated. An1cca's test was indicating OK, but I would like to redo this test to re check. Science at its best, thanks Jees 
|
|
|

dysfunctional word machine

Posts: 1831 Joined: 15-Mar-2014 Last visit: 11-Jun-2018 Location: at the center of my universe
|
Jees wrote:I've never worked with sodbicarb for basing before, I just pored some powder in and stirred long enough. It was like it wouldn't dissolve and pH wouldn't rise quick either. This is not they way to work with it I suppose. So I guess one has to make a hot saturated solution and drop that in? You mustn't heat a solution of sodium bicarbonate. When you do, it decomposes into sodium carbonate: 2 NaHCO 3 -dT-> Na 2CO 3 + CO 2 + H 2O The fizzing CO 2 bubbles are an indication of the decomposition happening.
|
|
|

DMT-Nexus member
Posts: 212 Joined: 16-Oct-2016 Last visit: 15-Jun-2023
|
Exactly, I synthesize my carbonate by heating the bicarbonate in an oven at 200°C for 2 hours. But solutions decompose as well.
|
|
|

DMT-Nexus member
Posts: 4031 Joined: 28-Jun-2012 Last visit: 05-Mar-2024
|
I begin to dislike sodBIcarb as a base to work with  But I think we have maybe something: if lye and ammonia renders the ZnXX into liquid if only added enough, then what about sodium carbonate? Why would it be excluded from that club theme? I am not chem savvy enough to smart that question out on a drawing board, so why not simply test it? To base with, I took a pan with a very low level demin water (200 ml ?) and brought that to (near) boiling temp, and added sodium carbonate until it really began to show signs of saturation, it can take quite a lot before that happens. This became my titration agent, super concentrated sodcarb water. I did not prefer dumping sodcarb powder in the testing vial because I wanted to see only ZnXX as solids and not potential undissolved sodcarb. In the vial 100 ml standard vinegar 7 or 8%, a plunge of Zn and let it near boiling in the microwave a few times (like that movie clip) and gave it a night rest. Filtered the unreacted zinc off. Start adding and at about 5.9 a very first mist appears. At 7 the ZnCO3 game really begins. At 9 the viscosity is highest. At 10 looks like same amount flocculate but the viscosity start to drop. At 10.20 the amount of flocculate is visibly less. At 10.30 looking beter. ... At 10.60 all clear  Can it be so simple? It would be good for others to repeat the test I suppose. Have I overlooked something? If this really works, one can make a Zn reduction conversion on freebase harmine+harmaline mix, or on harmaline alone, base with super concentrated sodcarb water till say maybe 11 (at least 10.60 or some higher, meter tolerances exist and this forces us to a margin). Filter, wash, done. No lye, no ammonia. For this experiment I needed give-or-take 75 ml super concentrated sodcarb water to reach 10.60 on a vial that started with 75 ml (I lost 25 ml on a pre-test). * * * Just another thought for food: Since it is easier to separate harmine from THH than to separate harmine from harmaline, I would prefer to reduce a mix of harmine+harmaline, and separate the unaltered harmine from the obtained THH later if wished. I hope to catch some sleep  Jees attached the following image(s):  01.jpg (100kb) downloaded 310 time(s). 02.jpg (92kb) downloaded 307 time(s). 03.jpg (77kb) downloaded 308 time(s). 04.jpg (95kb) downloaded 307 time(s). 05.jpg (81kb) downloaded 307 time(s). 06.jpg (81kb) downloaded 307 time(s). 07.jpg (85kb) downloaded 307 time(s). 08.jpg (84kb) downloaded 307 time(s). 09.jpg (78kb) downloaded 306 time(s). 10.jpg (82kb) downloaded 305 time(s). 11.jpg (83kb) downloaded 303 time(s).
|
|
|

DMT-Nexus member
Posts: 1893 Joined: 18-Jan-2008 Last visit: 26-Sep-2023
|
Interesting, on paper nothing other than Zinc carbonate should form which is insoluble...but hang on, thats in water not strongly basic solution...plot thickens.. Ok, so yes it's soluble in highly basic solutions "Solubility 0.001 G/100 G OF WATER AT 15 DEG C; SOL IN DIL ACIDS, ALKALIES & SOLN OF AMMONIUM SALTS. Budavari, S. (ed.). The Merck Index - Encyclopedia of Chemicals, Drugs and Biologicals. Rahway, NJ: Merck and Co., Inc., 1989., p. 1594" https://pubchem.ncbi.nlm...c_carbonate#section=OdorIts harmful to the environment so please dispose of responsibly.
|
|
|

DMT-Nexus member
Posts: 212 Joined: 16-Oct-2016 Last visit: 15-Jun-2023
|
Excellent work Jees! The flocculent/gel-like precipitate that forms from sodium carbonate could be a complex/double-salt ZnCO3/Zn(OH)2. This was mentioned by Pitubo in post #79. I really hope your extrapolations will prove to be correct Jees. Possibly however, the alkaloids that co-precipitate will attach to/envelop the preipitate making it more difficult for it to redissolve. I don't know, it's just that I wouldn't want my THH to get stuck in this precipitate  . In the meantime, I completed the reduction and precipitated THH in 3 different ways: 1) adding an excess ammonia 12% 2) precipitating zinc ions with bicarbonate, filtering, excess ammonia 3) precipitating zinc ions with bicarbonate, filtering, excess Na2CO3 The residues are drying right now, will keep you updated! What I did notice already was that: -again, the precipitate formed by bicarbonate basification was easy to filter -precipitating with carbonate after bicarbonate gave a less 'solid' precipitate than with ammonia and it also attached itself to the sides of the vessel. I'll check on my pictures to what pH I brought the solution (definitely more than 10,3). And YES, microscopy rules! Wow, I never thought harmaline could be so beautiful  . It's like taking a walk in the amazon jungle. Coincidence? And as for the THH: it's clearly not DHH, success! Will post some pictures later on as well.
|
|
|

DMT-Nexus member
Posts: 1263 Joined: 01-Jun-2014 Last visit: 10-Aug-2019
|
|
|
|

DMT-Nexus member
Posts: 4031 Joined: 28-Jun-2012 Last visit: 05-Mar-2024
|
I don't see much reasons to fear the zinc carbonate suddenly now. It is a natural occurring mineral ( wikipedia). This page is not extremely alarming. An1cca wrote:...I don't know, it's just that I wouldn't want my THH to get stuck in this precipitate... I think this is the biggest advantage to separate harmine from harmaline first before any reduction. It is the only way (attempts so far) to keep ZnOH or ZnCO3 precipitating away from precipitating alks. An1cca wrote:...Possibly however, the alkaloids that co-precipitate will attach to/envelop the preipitate making it more difficult for it to redissolve... That is still a question isn't it. In the best case it's not an issue at all, this has to be found out. How far did the pH went up under adding the sodBIcarb? Was there a layer undissolved at the botton? An1cca wrote:...precipitating with carbonate after bicarbonate gave a less 'solid' precipitate than with ammonia and it also attached itself to the sides of the vessel... Suppose your sodBIcarb was not able to precipitate all ZnOH/ZnCO3 complex? I've not been able to test it myself due that former post experiment. I do like to know how pH was after the sodcarb. Nice works, love it 
|
|
|

DMT-Nexus member
Posts: 212 Joined: 16-Oct-2016 Last visit: 15-Jun-2023
|
So this is what I did: The setup from post #82 was left to stand for 24h after which the unreacted zinc was filtered off (dry weight: 1998mg, precipitate 1 on pic1). After washing and rinsing, the total volume became 210ml (more or less as in VDS's article). This solution was divided in 2: 1)70ml were treated with ammonia 12%. Clouding was observed as from pH 5,2 that didn't intensify much until at pH 9,5 the real precipitation and pH-depression began (+/- 9ml). Then another 10ml of ammonia were added that brought the pH up to 10,72. The solution was filtered to give precipitate 2 (710mg). 2)To the other 140ml there was added sodium bicarbonate powder. after adding 4,650g clouding appeared and the pH read 6,11. More bicarbonate was added that raised the clouding and the pH up to 7,58 (12 grams). This solution was filtered (zinc salts + some bicarbonate?): precipitate 3 (still drying) and divided in two parts of 70ml each: 3) 70ml were treated with ammonia 12%. after 0,6ml, the pH read 8,87 and clouding appeared. The pH was brought to 10,30 with 7ml of ammonia in total. After filtering and drying, the residue precipitate 4 weighed 706mg. 4) To the other 70ml, there was added a concentrated solution of sodium carbonate (8g/40ml water). This brought the pH to 10,8. After filtering, washing with Na2CO3 0,5% solution and drying, precipitate 5 weighed 674mg. It is powdery, had no 'gel-properties' and dried as quick as the precipitate 4. So, it seems THERE IS AN AMMONIA-FREE ROUTE. In this case, because the experiment had already started after Jees's post, both sodium carbonate and bicarbonate were used. But hopefully, a carbonate-only route will be possible as well... Considering the absolute simplicity of this method, the yields are just fine. Around 70% for the ammonia routes and slightly less for the carbonate-route. About the pics: pic2: the mother liquor after reduction definitely has a lighter color (check post #82) pic3: sodium bicarbonate was added up to pH 7,58 pic4: UV image at start of reduction pic5: UV image at end of reduction (DHH on arm) pic6: circular paper chromatogram harmine (dried acetate, eluent IPA80% Acetic acid 20%) pic7: circular paper chromatogram harmaline pic8: circular paper chromatogram THH pic9: in situ recrystallization of mother liquor at start of reduction pic10: in situ recrystallization of mother liquor at end of reduction pic11: THH clusters precipitated with ammonia in reaction vessel pic12: THH detail of pic 10 The microscopic pictures aren't state of the art, I know. I did my best at catching something with my phone and a children's microscope..... And...for those who have not given up by now: a special treat: toroidal THH.HCl vortices while drops of saturated NaCl-solution fall into the dissolved THH-residues, Manske-style, enjoy  !
|
|
|

DMT-Nexus member
Posts: 4031 Joined: 28-Jun-2012 Last visit: 05-Mar-2024
|
Some bits: Maybe more than watching, steering thoughts and guiding hands  DreaMTripper wrote:...Its [ZnCO3 or ZnOH] harmful to the environment so please dispose of responsibly. If you have some information of that I would like to read it gladly. An1cca wrote:...And YES, microscopy rules! Wow, I never thought harmaline could be so beautiful... What X factor should a microscope have at least to see the harmala crystals well? There are so many to chose from. The Bresser BioDiscover 20X-1280X that VDS used looks like a cost friendly and attractive entry level. pitubo wrote:You mustn't heat a solution of sodium bicarbonate. When you do, it decomposes into sodium carbonate: 2 NaHCO3 -dT-> Na2CO3 + CO2 + H2O .The fizzing CO2 bubbles are an indication of the decomposition happening. I was thinking of heating water to say 80 deg C and poring the sodBIcarb in, would it also start to transform in sodcarb on that modest level of heating? I would do that to minimize the needed volume of basing liquid. 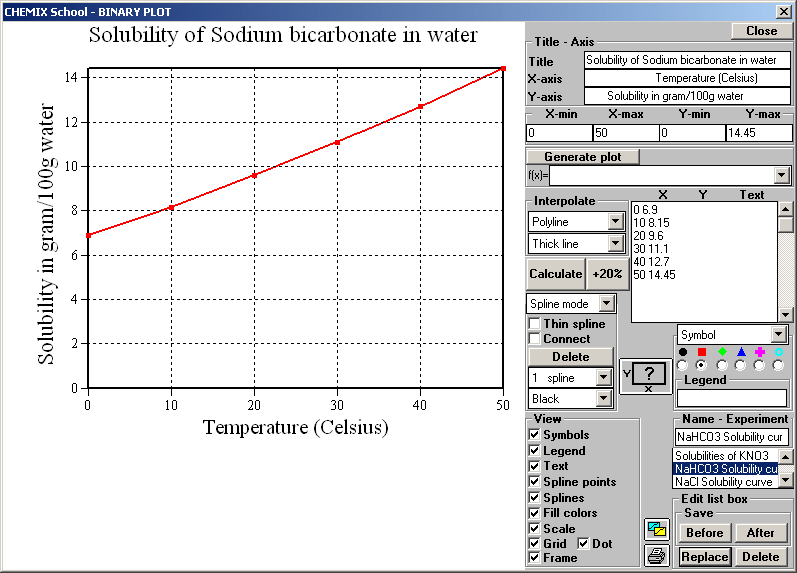
|
|
|

DMT-Nexus member
Posts: 4031 Joined: 28-Jun-2012 Last visit: 05-Mar-2024
|
An1cca wrote:So this is what I did:... In the process of digesting all this  Yum yum 
|
|
|

DMT-Nexus member
Posts: 1893 Joined: 18-Jan-2008 Last visit: 26-Sep-2023
|
Great pics An1cca marvelous work! "...Its [ZnCO3 or ZnOH] harmful to the environment so please dispose of responsibly." If you have some information of that I would like to read it gladly." I just saw a note on the bottom of a summary thought it was worth mentioning and considering, effects probably nominal at these ammounts. Attached a pdf that has a comprehensive assessment.
|